One gram-mole each of CO 2 , O 2 , and N 2 are fed to a
Question:
One gram-mole each of CO2, O2, and N2 are fed to a batch reactor and heated to 3000K and 5.0atm. The two reactions given here proceed to equilibrium (also shown are the equilibrium constants at 3000 K). Calculate the equilibrium composition (component mole fractions) of the reactor contents. [Suggestion: Express K1 and K2 in terms of the extents of the two reactions ?1 and ?2. (See Section 4.6d) Then use an equation-solving program or a trial-and-error procedure, such as the Newton-Raphson method (Appendix A.2), to solve for ?1 and ?2, and use the results to determine the equilibrium mole fractions.]
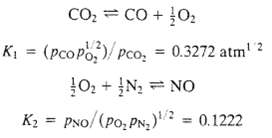
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau
Question Posted:





