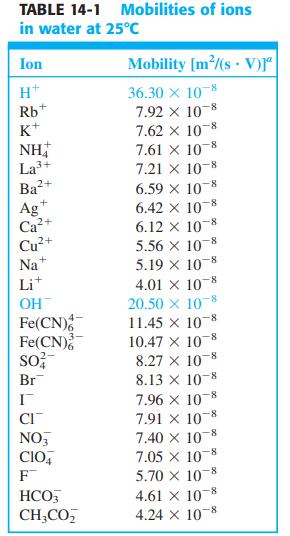
Refer to the footnote in Table 14-1. How many seconds will it take for (a) H +
Question:
Refer to the footnote in Table 14-1. How many seconds will it take for (a) H+ and (b) NO-3 to migrate a distance of 12.0 cm in a field of 7.80 × 103 V/m?
Transcribed Image Text:
TABLE 14-1 Mobilities of ions in water at 25°c Ion Mobility [m/(s V)r" 36.30 x 108 7.92 x 10-8 7.62 x 10 7.61 X 10 8 7.21 x 10-8 H* Rb K+ NH La3+ Ba2+ 6.59 X 10 6.42 x 10-8 6.12 x 10 5.56 X 10-8 +, Ag Ca²+ Cu+ Na* 8- 5.19 x 10 4.01 x 10-8 20.50 x 10 11.45 x 10-8 10.47 X 10-8 8.27 x 10 8.13 x 10-8 Lit OH Fe(CN) Fe(CN) so; Br 7.96 X 10 7.91 x 10 7.40 X 108 7.05 x 10 5.70 x 10-8 CI NO, CIO, F 4.61 X 10-8 - 4.24 X 10-8 HCO, CH;CO,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (12 reviews)
Velocity mobility field 3630 10 8 m 2 s V 780...View the full answer

Answered By

Pranav Makode
I am a bachelor students studying at professor ram meghe institute of technology and research. I have a great experience of being an expert. I have worked as an expert at helloexperts and solvelancer as a part time job. I have also worked as a doubt solver at ICAD SCHOOL OF LEARNING, which is in Amravati city. I have also worked as an Freelancer.
I have great experience of helping students, as described above. I can help any students in a most simple and understandable way. I will not give you have any chance for complaint. You will be greatfull to accept me as an expert.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
1. How many seconds would it take a 7.00-hp motor to raise a 475-lb boiler to a platform 38.0 ft high? 2. How long would it take a 950-W motor to raise a 360-kg mass to a height of 16.0 m?
-
How many seconds are there in a solar year (365.24 days)?
-
How many time constants will it take for a charged capacitor to be discharged to one-fourth of its initial stored energy?
-
Write a method leve1Order() that prints BST keys in level order: first print the root; then the nodes one level below the root, left to right; then the nodes two levels below the root (left to...
-
Define a provision and give an example.
-
In March 2012, the spread betting firm, Worldspreads Ltd, applied to go into administration. The decision was made when the company realized its cash balance of 16.6 million could not meet its...
-
9-9. Por qu los mercadlogos usan mapas perceptuales en las decisiones de posicionamiento de un producto?
-
Novelis, Incorporated is the worlds leading rolledaluminum products producer. Items 1. through 3. below provide descriptions of issues involving asset impairments derived from the companys footnote...
-
Part 3 Prepare a monthly Income statement. Use Tables A and C. (Note: From this part and on consider that every baked bread will 3 Revenue 21 Cost of Goods Sot Gro Men 5 directed Cuts 41 5 Income...
-
If your consumption of toothpaste produces positive externalities for your neighbors (which you ignore), then you are consuming less toothpaste than the Pareto optimal quantity. True False
-
Which side of the liquid junction 0.1 M KNO3 | 0.1 M NaCl will be negative?
-
Suppose that an ideal hypothetical cell such as that in Figure 13-7 were set up to measure E for the half-reaction . (a) Calculate the equilibrium constant for the net cell reaction. (b) If there...
-
1. Per the city's schedule of long-term obligations, what is the total long-term obligation for both governmental and business-type activities? Does this amount reconcile with the long-term...
-
Thomson Company's income statement for the year ended December 31, 20X4, reported net income of $360,000. The financial statements also disclosed the following information: Depreciation $60,000...
-
Based on past experience, Maas Corporation (a U.S.-based company) expects to purchase raw materials from a foreign supplier at a cost of 1,800,000 francs on March 15, 2024. To hedge this forecasted...
-
Suppose that laws are passed banning labor unions and that resulting lower labor costs are passed along to consumers in the form of lower prices. Assume that the U.S. economy was in long-run...
-
What's wrong with the following statement? "Because the digits 0, 1, 2,....9 are the normal results from lottery drawings, such randomly selected numbers have a normal distribution." Choose the...
-
Matching Question Drag and drop various responsibilities of employers that are related to workplace values against the corresponding values. Drag and drop application. Justice Justice drop zone...
-
Identify three issues that a general contractor would be concerned about when negotiating subcontracts.
-
A routine activity such as pumping gasoline can be related to many of the concepts studied in this text. Suppose that premium unleaded costs $3.75 per gal. Work Exercises in order. Use the...
-
A 3.455-g sample of a mixture was analyzed for barium ion by adding a small excess of sulfuric acid to an aqueous solution of the sample. The resultant reaction produced a precipitate of barium...
-
A tanker truck carrying 5.0 103 kg of concentrated sulfuric acid solution tips over and spills its load. If the sulfuric acid is 95.0% H2SO4 by mass and has a density of 1.84 g/mL, how many...
-
A sample of 5.53 g of Mg(OH)2 is added to 25.0 mL of 0.200 M HNO3. (a) Write the chemical equation for the reaction that occurs. (b) Which is the limiting reactant in the reaction? (c) How many moles...
-
*please calculate irr in excel
-
Which of the following would not be a period cost? Research and development Direct materials Office supplies Advertising costs
-
\ table [ [ Activity Cost Pool,Activity Measure,Total Cost,Total Activity ] , [ Machining , Machine - hours,$ 3 3 0 , 0 0 0 , 1 5 , 0 0 0 MHs ] , [ Machine setups,Number of setups,$ 3 0 0 , 0 0 0 , 5...

Study smarter with the SolutionInn App


