Repeat Problem 68 with a diatomic gas. B.
Question:
Repeat Problem 68 with a diatomic gas.
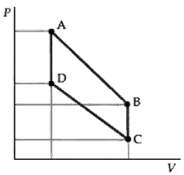
Transcribed Image Text:
B.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 77% (9 reviews)
Proceed as in Problem 68 with C v 5 R and 14 The pressures volumes and temperatures are as sh...View the full answer

Answered By

AJIN KURIAKOSE
I HAVE ELECTRONICS ENGINEERING DEGREE..AND MY AREA OF INTEREST IS MATHEMATICS,CONTROL SYSTEM,NETWORK,DIGITAL
4.70+
21+ Reviews
32+ Question Solved
Related Book For 

Fundamentals of Ethics for Scientists and Engineers
ISBN: 978-0195134889
1st Edition
Authors: Edmund G. Seebauer, Robert L. Barry
Question Posted:
Students also viewed these Thermodynamics questions
-
Repeat parts (a) and (b) of Problem 68 with hre = 2 x 10-4 and compare results. a. Determine Zi and Zo. b. Calculate Av and Ai 18 V 2.2 68 k - 180 Z, 2.75 k2 25 Zi
-
A gas mixture is found to contain two diatomic A and B species for which the partial pressures of both are 0.05065 MPa (0.5 atm). This mixture is to be enriched in the partial pressure of the A...
-
Repeat Problem 68 when the proportion of the population having a value of less than x is equal to 1 ex. Problem 68 The number of accidents that a person has in a given year is a Poisson random...
-
In Exercises, use the alternative form of the derivative to find the derivative at x = c (if it exists). f(x) = 1 x + 4' c = 3
-
Forsyth Company manufactures one product, it does not maintain any beginning or ending inventories, and its uses a standard cost system. During the year, the company produced and sold 10,000 units at...
-
Iodine, I 2 , reacts with thiosulfate ions, S 2 O 3 2 to form iodide ions, I , and tetrathionate ions, S 4 O 6 2 I 2 +S 2 O 3 2 2I + S 4 O 6 2 a. State the oxidation number of each sulfur atom in:...
-
In which of the following areas does the IASB allow firms to choose between a benchmark treat ment and an allowed alternative treatment? LO4 a. Measuring property, plant, and equipment subsequent to...
-
The president of Kelly Company is interested in determining how effective the company's new controller has been in controlling cash on hand. You have the following information available from the...
-
SweetFish Corp. issued bonds with a par value of $895,000 and a five-year life on May 1, 2020. The contract interest rate is 9.00%. The bonds pay interest on October 31 and April 30. They were issued...
-
Brookhurst Company (a U.S.-based company) established a subsidiary in South Africa on January 1, Year 1, by investing 300,000 South African rand (ZAR) when the exchange rate was US$0.09/ZAR 1. On...
-
Repeat Problem 67 with a diatomic gas. B.
-
An ideal gas of n mol is initially at pressure P1, volume V1, and temperature Th. It expands isothermally until its pressure and volume are P2 and V2. It then expands adiabatically until its...
-
The ocean possesses enormous numbers of molecules, all with kinetic energy. Can this energy be extracted and used as a power source? Defend your answer.
-
For the demand equation, express the total revenue R as a function of the price p per item. R(p) q=-6p+ 600 Sketch the graph of the resulting function. 20000 19000 18000 17000 16000 O 15000 14000...
-
Kosovski Company is considering Projects S and L, whose cash flows are shown below. These projects are mutually exclusive, equally risky, and repeatable. The WACC is 11.50%. Year: 0 1 2 3 4 CF for S:...
-
What is among the most important things you should do in a negotiation? What is among the most important things you should do in a negotiation? Try to get your way on as many issues as possible. Find...
-
analyze the following column values and answer question: Value Value Label Frequency Percentage Weighted Percentage 1 - 87 Number of children Notes: _ _ = Number of children 113,819 25.78 36.41 88...
-
Reflect on the following questions. Post your response to the discussion board. Post your discussion post by Thursday . 1 peer response due by Sunday. This discussion has two parts: 1) Mediators and...
-
How is the Inventory account on the balance sheet linked to the income statement? How does the allocation of the capitalized inventory cost affect the financial statements?
-
A 6-lb shell moving with a velocity ?? v0k explodes at point D into three fragments which hit the vertical wall at the points indicated. Fragments A, B, and C hit the wall 0.010 s, 0.018 s, and 0.012...
-
It has become common to replace the cataract-clouded lens of the eye with an internal lens. This intraocular lens can be chosen so that the person has perfect distant vision. Will the person be able...
-
A physics student immerses one end of a copper rod in boiling water at 100C and the other end in an ice-water mixture at 0C. The sides of the rod are insulated. After steady-state conditions have...
-
To heat 1 cup of water (250 cm3) to make coffee, you place an electric heating element in the cup. As the water temperature increases from 20C to 65C, the temperature of the heating element remains...
-
An object of mass m1 specific heat capacity C1 and temperature T1 is placed in contact with a second object of mass m2, specific heat capacity C2, and temperature T2 > T1 . As a result, the...
-
Your company produces a health magazine. Its sales data for 1 - year subscriptions are as follows: Year of Operation Subscriptions Sold % Expired at Year End 2 0 2 0 $ 3 0 0 , 0 0 0 5 0 2 0 2 1 $ 6 4...
-
The adjusted trial balance for Tybalt Construction on December 3 1 of the current year follows. TYBALT CONSTRUCTION Adjusted Trial Balance December 3 1 Number Account Title Debit Credit 1 0 1 Cash $...
-
( US$ millions ) 1 2 / 3 1 / 2 0 1 4 1 2 / 3 1 / 2 0 1 3 1 2 / 3 1 / 2 0 1 2 1 2 / 3 1 / 2 0 1 1 Net income $ 1 4 , 4 3 1 $ 1 2 , 8 5 5 $ 1 0 , 7 7 3 $ 9 , 7 7 2 Depreciation 3 , 5 4 4 2 , 7 0 9 1 ,...

Study smarter with the SolutionInn App


