Samples A and B are at different initial temperatures when they are placed in a thermally insulated
Question:
Samples A and B are at different initial temperatures when they are placed in a thermally insulated container and allowed to come to thermal equilibrium. Figure a gives their temperatures Z versus time t. Sample A has a mass of 5.0 kg; sample B has a mass of 1.5 kg. Figure b is a general plot for the material of sample B. It shows the temperature change AZ that the material undergoes when energy is transferred to it as heat Q. The change ?T is plotted versus the energy Q per unit mass of the material, and the scale of the vertical axis is set by ?Ts = 4.0 Co. What is the specific heat of sample A?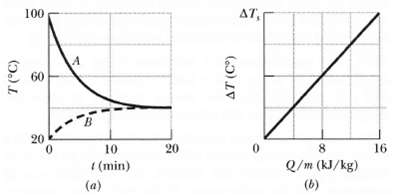
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals of Physics
ISBN: 978-0471758013
8th Extended edition
Authors: Jearl Walker, Halliday Resnick
Question Posted:





