Select indicators from Table 15-2 that would be suitable for finding the end point in Figure 15-3.
Question:
Select indicators from Table 15-2 that would be suitable for finding the end point in Figure 15-3. What color changes would be observed?
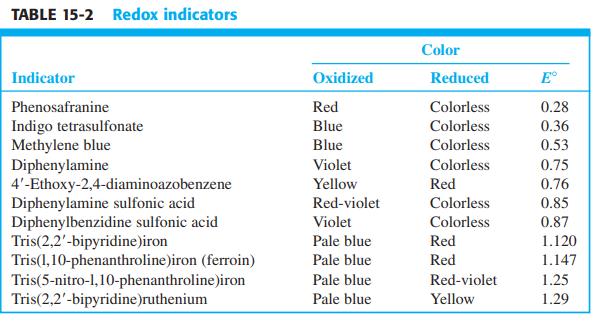
Figure 15-3
Transcribed Image Text:
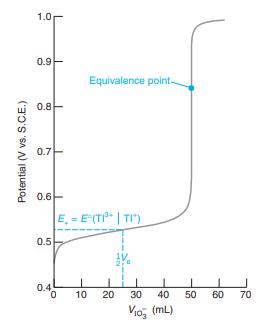
TABLE 15-2 Redox indicators Color Indicator Oxidized Reduced E° Phenosafranine Red Colorless 0.28 Indigo tetrasulfonate Methylene blue Diphenylamine 4'-Ethoxy-2,4-diaminoazobenzene Diphenylamine sulfonic acid Diphenylbenzidine sulfonic acid Tris(2,2'-bipyridine)iron Tris(1,10-phenanthroline)iron (ferroin) Tris(5-nitro-l,10-phenanthroline)iron Tris(2,2'-bipyridine)ruthenium Blue Colorless 0.36 Blue Colorless 0.53 Violet Colorless 0.75 Yellow Red 0.76 Red-violet Colorless 0.85 Violet Colorless 0.87 Pale blue Red 1.120 Pale blue Red 1.147 Pale blue Red-violet 1.25 Pale blue Yellow 1.29
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
Diphenylamine sulfonic acid colo...View the full answer

Answered By

Nandana Wijayarathna
I am a highly experienced writer in several areas,
Business management
Information technology
Business administration
Literature
Biology
Environmental science
History
4.50+
161+ Reviews
399+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
Name the following polymer(s) that would be suitable for the fabrication of cups to contain hot coffee: polyethylene, polypropylene, poly (vinyl chloride), PET polyester, and polycarbonate. Why?
-
Name the following polymer(s) that would be suitable for the fabrication of cups to contain hot coffee: polyethylene, polypropylene, poly(vinyl chloride), PET polyester, and polycarbonate. Why?
-
Several material combinations and structures have been described in this chapter. In relative terms, identify those that would be suitable for applications involving one of the following: (a) Very...
-
Consider a situation with J identical firms that have marginal abatement cost functions for j=1,,J. The marginal damage function is equal to D'(E)=d.EDetermine the optimal allocation and the optimal...
-
At December 31, 2014, Torrealba Company reported the following as plant assets. During 2015, the following selected cash transactions occurred. April 1 Purchased land for $1,200,000. May 1 Sold...
-
AP Suppose the following data are derived from the 2025 financial statements of Southwest Airlines. (All dollars are in millions.) Southwest has a December 31 year-end. Instructions a. After...
-
Identify the following terms: PV, EV, and AC. Why are these terms important? How do they relate to one another?
-
Seth Feye established Reliance Financial Services on July 1, 2019. Reliance Financial Services offers financial planning advice to its clients. The effect of each transaction and the balances after...
-
b. Compute not present value for wach project assed on net presect vilue, which project a preforred? Cemplete this eatstion ly enterlag vour answers in the tabs belew. so 2 decimal placen. Gonzalez...
-
1. In the mix of premiums the Porterfields can spend, how should Adam and Cassie rank Adam's insurance needs for the seven types of coverage offered? What factors should they consider? 2. Should Adam...
-
Ascorbic acid (0.010 0 M) was added to 10.0 mL of 0.020 0 M Fe 3+ in a solution buffered to pH 0.30, and the potential was monitored with Pt and saturated Ag | AgCl electrodes. Dehydroascorbic acid +...
-
Would tris(2,2 -bipyridine)iron be a useful indicator for the titration of Sn 2+ with Mn(EDTA) - ? (Hint: The potential at the equivalence point must be between the potentials for each redox couple.)
-
A survey indicated that companies with fewer than 1,000 employees are expected to increase their spending by 20.4 %. Form a 99 % confidence interval for the unknown mean increase, assuming that the...
-
The copper coil placed inside a stove with the purpose of heating water that flows through the coil. The coil is made from copper tube with an OD of 1 2 . 7 0 mm and ID of 1 1 . 0 8 mm . Water enters...
-
Confidence Levels Given specific sample data, such as the data given in Exercise 1, which confidence interval is wider: the 95% confidence interval or the 80% confidence interval? Why is it wider?
-
Yellow M&Ms Express the confidence interval (0.0847, 0.153) in the form of P - E < p < p + E. 12. Blue M&Ms Express the confidence interval 0.255 (+-) 0.046 in the form of P - E < p < p + E.
-
An ideal, noble gas with a mass of 97.2 g at 25 C and a pressure of 608 torr has a volume of 22.7 L. 1. What is the pressure (in atm)? SHOW ALL WORK. 2. What is R (number and units)? 3. What is the...
-
A drug is used to help prevent blood clots in certain patients. In clinical trials, among 4705 patients treated with the drug, 170 developed the adverse reaction of nausea. Construct a 95% confidence...
-
Why do community values matter to a developer? Give an example of a specific community value that might impact a project.
-
Read Case Study Google: Dont Be Evil Unless and answer the following: Given its mission of providing information to the world, should Google censor searches in China?
-
Why is sample dropped into the preheated furnace before the oxygen concentration reaches its peak in Figure 26-7?
-
Write a balanced equation for the combustion of benzoic acid, C6H5CO2H, to give CO2 and H2O. How many milligrams of CO2 and of H2O will be produced by the combustion of 4.635 mg of benzoic acid?
-
Combustion analysis of a compound known to contain just C, H, N, and O demonstrated that it is 46.21 wt% C, 9.02 wt% H, 13.74 wt% N, and, by difference, 100 - 46.21 - 9.02 -13.74 = 31.03% O. This...
-
Comparative financial statements for Weller Corporation, a merchandising company, for the year ending December 31 appear below. The company did not issue any new common stock during the year. A total...
-
Mrquered Mrquered
-
You plan to invest $10,00 today in an investment account earning 5% interest. You then plan to invest an additional $1,000 into this account each year for the next twenty years. How much money will...

Study smarter with the SolutionInn App


