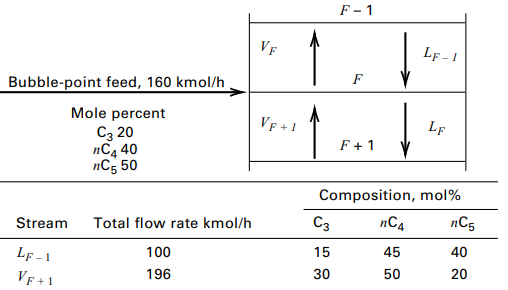
Streams entering stage F of a distillation column are shown in Figure. What is the temperature of
Question:
Streams entering stage F of a distillation column are shown in Figure. What is the temperature of stage F and the compositions and amounts of streams VF and LF if the pressure is 785kPa for all streams? Use a simulation computer program to obtain the answers.
Transcribed Image Text:
F - 1 VF Lf - 1 Bubble-point feed, 160 kmol/h Mole percent C3 20 пСд40 nC5 50 VF + 1 LF Composition, mol% nC4 пС5 Сз Total flow rate kmol/h Stream 40 45 15 100 Lf - 1 20 50 30 196 VF + 1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)
Using the SRK method for Kvalues with the CHEMCAD process simulator ...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
In figure what is the net electric potential at point P due to the four particles if V = 0 at infinity, q = 5.00fC, and d =4.00cm? +9
-
The wobble rules for tRNA-mRNA pairing are shown in Figure 13.12. If we assume that the tRNAs do not contain modified bases, what is the minimum number of tRNAs needed to efficiently recognize the...
-
What is the temperature of 5F in degrees Rankine?
-
Write a program that takes three double command-line arguments x, y, and z, reads from standard input a sequence of point coordinates (xi, yi, zi), and prints the coordinates of the point closest to...
-
Using the same information from BE8-10 above, prepare the journal entries that Red River Enterprises will record regarding the issue of the note and the receipt of the merchandise inventory. Red...
-
Given the following, calculate net income: Dept. 1 Dept. 2 Net Sales $39,000 $43,000 Costof Goods Sold 24,000 30,000 Operating Expenses $16,400 Income Tax Expense, 30% rate
-
Use the PewSocialMedia dataset to run a set of nested regression models, using the Index of Cellphone Uses as your dependent variable. In the first variable, use only the age variable. In the second...
-
Wefald Company issued $600,000, 10-year, 10 percent bonds on January 1, 2011. The bonds sold for $580,000. Interest is payable semiannually each June 30 and December 31. Record the sale of the bonds...
-
Cowboy Company's sales employees earn a total of $41,000 per month and are paid on the last working day of the month. Each employees wages are subject to FICA Social Security taxes of 6.2% and...
-
The responses of 1019 adults who were asked how much money they think they will spend on Christmas gifts in a recent year $1000 or more: 306 $250999: 336 Less than $250: 234 Not sure: 51 None/do not...
-
As shown in Figure, a hydrocarbon mixture is heated and expanded before entering a distillation column. Calculate, using a simulation computer program, the mole percent vapor phase and vapor and...
-
Flash adiabatically, across a valve, a stream composed of the six hydrocarbons given below. The feed upstream of the valve is at 250?F and 500 psia. The pressure downstream of the valve is 300 psia....
-
What is putcall parity and why does it hold? Could you apply the parity formula to a call and put with different exercise prices?
-
Locate a scholarly article relevant to how to present your financial plan for opening a Roller Skating Rink (from your draft business plan) to a lending institution--and describe your strategy for...
-
How would you expect seasonal fluctuations in demand to affect a rental company's decisions about pricing rented products such as wedding dresses or convertible cars? In terms of pricing principles,...
-
Do we drive technology, or does technology drive us? If technology drives us, what are the risks? The other side of the coin would be that we are able to stay ahead of technological transformations....
-
How do you explain the differences between the two analyses and what are the implications of using the BCG matrix in practice?
-
How do leadership styles, such as transformational leadership, shared leadership, and servant leadership, impact team dynamics, member motivation, and overall team effectiveness ?
-
Be able to perform an analysis based on horizontal and/or vertical analysis of financial statements.
-
A stock has had returns of 8 percent, 26 percent, 14 percent, 17 percent, 31 percent, and 1 percent over the last six years. What are the arithmetic and geometric average returns for the stock?
-
What are some of the external factors that can influence an organizations workforce plans?
-
In the rate-based model, is the assumption of phase equilibrium used anywhere? If so, where? Is it justified?
-
What is meant by ion exchange? How does ion exchange differ from deionization?
-
Porous particles of activated alumina have a BET surface area of 310 m 2 /g, p = 0:48, and p = 1.30 g/cm 3 . Determine: (a) V p in cm 3 /g; (b) s , in g/cm 3 ; and (c) d p in A.
-
Al preparar el estado de resultados pro forma, cules de las siguientes partidas se deducen de las utilidades brutas para llegar a las ganancias despus de impuestos? Pregunta de seleccin mltiple....
-
Lawson Inc. is expanding its manufacturing plant, which requires an investment of $4 million in new equipment and plant modifications. Lawson's sales are expected to increase by $3 million per year...
-
20 On January 1, Year 1, X Company purchased equipment for $80,000. The company estimates that the equipment will have a useful life of 10 years and a residual value of $5,000. X Company depreciates...

Study smarter with the SolutionInn App


