Consider an (alpha) particle, which is the nucleus of ({ }_{2}^{4} mathrm{He}) atomic mass (4.002603 mathrm{u}). (a)
Question:
Consider an \(\alpha\) particle, which is the nucleus of \({ }_{2}^{4} \mathrm{He}\) atomic mass \(4.002603 \mathrm{u}\).
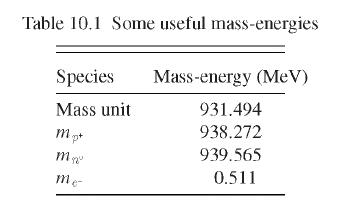
(a) Use the data in Table 10.1 to calculate the binding energy per nucleon.
(b) Release of an \(\alpha\) particle from a nucleus is a common mode of decay for many radioactive heavy nuclei. Explain why the \(\alpha\) decay of \({ }_{88}^{226} \mathrm{Ra}\) is more likely than decay by release of a \(p^{+}\). The atomic mass of \({ }_{87}^{225} \mathrm{Fr}\) is 225.025572 .
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

An Introduction To Groups And Their Matrices For Science Students
ISBN: 9781108831086
1st Edition
Authors: Robert Kolenkow
Question Posted: