Acrylic acid at 89C and 0.16 kPa ( = 970 kg/m 3 , = 0.46 cP)
Question:
Acrylic acid at 89°C and 0.16 kPa (ρ = 970 kg/m3, μ = 0.46 cP) leaves the bottom of a distillation column at a rate of 1.5 L/s.
The bottom of a distillation column may be assumed to behave like a tank containing vapor and liquid in equilibrium at the temperature and pressure of the exit stream. The liquid must be pumped to a railroad heading supply tank 4.0 m above the liquid level in the distillation column, where the pressure must be 116 kPa. The liquid level at the bottom of the distillation column is 3.5 m above the pump suction line, and the frictional head loss for the suction line including the tank exit is 0.2 m of acrylic acid. There is a cooler after the pump with a pressure drop of 3.5 m of acrylic acid. The discharge line is 1.5-in, schedule-40, commercial-steel pipe, with an equivalent length of 200 m. The entire process may be assumed to be isothermal at 89°C. The problem at hand is whether this system can be scaled up by 20%. The plots required for this analysis are in Figure P19.71.
Based on a pump/system curve analysis, can this portion of the process be scaled up by 20%? If not, what is the maximum scale-up percentage?
Based on an NPSH analysis, is it good operating policy for this portion of the process to be scaled up by 20%? If not, what is the maximum recommended scale-up percentage?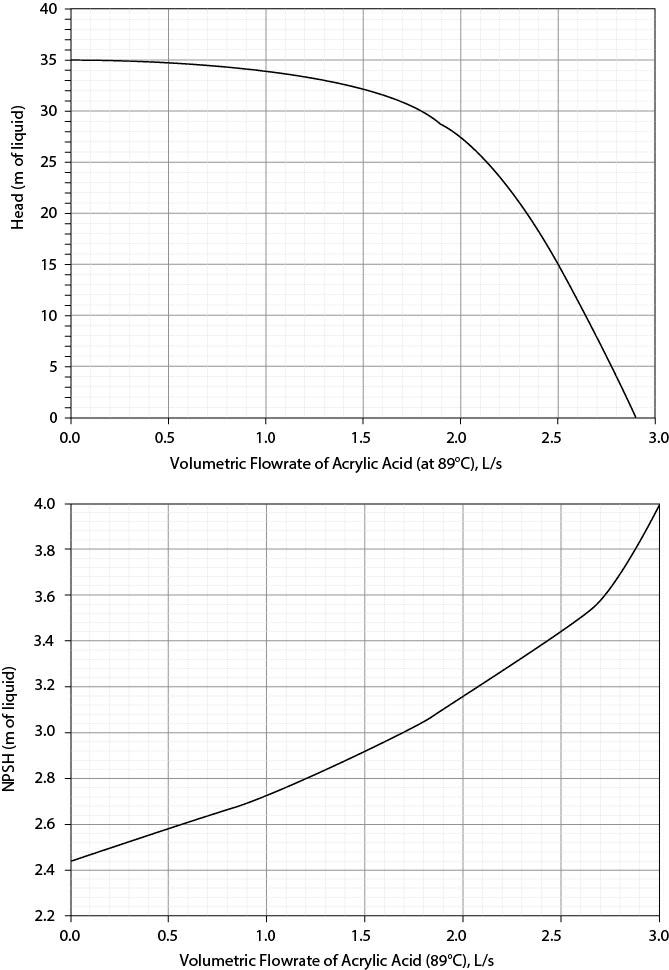
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting





