Consider the dehydration of isopropyl alcohol (IPA) to yield acetone and hydrogen: This reaction is endothermic, with
Question:
Consider the dehydration of isopropyl alcohol (IPA) to yield acetone and hydrogen: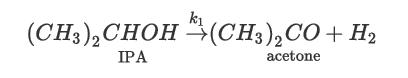
This reaction is endothermic, with a heat of reaction of 57.2 kJ/mol. The reaction is kinetically controlled and occurs in the vapor phase over a catalyst. The reaction kinetics for this reaction are first order with respect to the concentration of alcohol and can be estimated from the following equation:![-Ea RT [K] -TIPA = ko exp |- R CIPA kmol/m (catalyst)/s](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/4/4/2/186654b6e0a3c16a1699442184507.jpg)
where Ea = 72.38 MJ/kmol, ko = 1.931×105 m3 (gas)/m3 (reactor)/s, and cIPA has units of kmol/m3 (gas).
The feed to the reactor is 87 wt% IPA in water at 240°C and 2 bar. Spherical catalyst particles (5 mm in diameter) are placed in 50 mm ID tubes of length 6 m. The heat transfer coefficient on the tube side of the reactor is limiting. A heat transfer medium (HTM) is available as part of a heating loop that supplies the HTM to the reactor shell at 400°C.
Properties of the heat transfer fluid are
Properties of the process gas are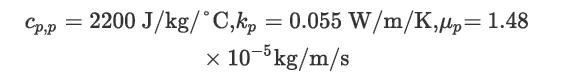
Properties of the catalyst are![]()
1. Determine the conversion of alcohol to acetone assuming that the HTM flows cocurrently with the process gas. Assume that the HTM flows at a rate of 200,000 kg/h, the inlet flowrate to the reactor is 8,000 kg/h of IPA and water, and the reactor has a total of 1600 tubes arranged on a square pitch with the tube centers 75 mm apart.
2. Sketch the temperature concentration profiles in a single reactor tube.
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting




