Cumene (isopropyl benzene) is manufactured from propylene and benzene. Most of the worlds supply of cumene is
Question:
Cumene (isopropyl benzene) is manufactured from propylene and benzene. Most of the world’s supply of cumene is directly converted into phenol, which is a raw material for a variety of products such as plasticizers.
Acetone is also manufactured as a by-product of phenol manufacture. The separation section of a cumene process consists of a flash, to remove the unreacted propylene, followed by a distillation column to separate the benzene and produce purified cumene as the bottom product.
1. For this problem, consider that the flash contains only propylene and benzene and operates at 3 atm and 100°C. The feed contains 80 mol%
benzene and 20 mol% propylene. On a basis of 200 kmol/h feed, determine the flowrates of vapor and liquid leaving the flash.
The feed to the distillation column is 60% liquid at 200 kmol/h containing 48 mol% benzene. It is necessary to produce 99 mol% cumene in the bottom product and 98 mol% benzene in the top product for recycle. The distillation column contains a total condenser and a partial reboiler. The column operates at an average pressure of 3 atm.
2. Determine the flowrates of the distillate and the bottoms.
3. For a reflux ratio of 0.85, calculate all internal flows.
4. Estimate the heat loads (kJ/h) on the condenser and reboiler.
5. Estimate the column efficiency and determine the actual number of trays, the actual number of stages, and the actual feed location. There are 10 equilibrium stages, with feed on Stage 4.
6. Design a sieve tray column for 75% of flooding, with 85% active tray area and with 18-in tray spacing. Specify the column diameter and the actual column height. Ignore the surface tension correction. It is up to you to determine whether to design for the top section or for the bottom section.
You should justify your choice.
7. Estimate the pressure drop (kPa) in the column if the trays have 6-in weirs.
8. Design a packed column for 75% of flooding with 2-in, ceramic Raschig rings. It is up to you to determine whether to design for the top section or for the bottom section. You should justify your choice.
9. There is an existing column with the correct number of trays with a 2 m diameter and 85% active area. Will this column work? Explain why or why not. Your answer must be supported with calculations. Could anything be done to make this column work? If so, what are the consequences?
10. The original column is now built and operational, and the feed is 60%
liquid. Due to a reactor upset, it is now necessary to accept feed, still 60%
liquid, at the same rate but containing 50 mol% benzene, with the remainder cumene. It is still necessary to produce 99 mol% cumene at the same rate from the bottom of this column. You assign your summer intern the job of determining new operating conditions. The answer you get back is to operate at a reflux ratio of 0.75. Will the column operate at these conditions?
Data: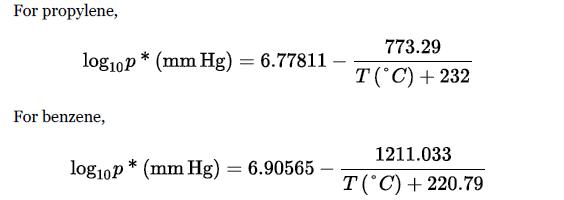
Average column relative volatility = 6
Feed viscosity = 0.3 cP
Benzene density = 800 kg/m3
Cumene density = 700 kg/m3
Benzene heat of vaporization = 3.07 × 104 kJ/kmol
Cumene heat of vaporization = 3.81 × 104 kJ/kmol
Estimated top temperature = 56°C
Estimated bottom temperature = 178°C
Benzene viscosity = 0.4 cP
Cumene viscosity = 0.3 cP
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting





