The formation of styrene via the dehydrogenation of ethylbenzene is a highly endothermic reaction. In addition, ethylbenzene
Question:
The formation of styrene via the dehydrogenation of ethylbenzene is a highly endothermic reaction. In addition, ethylbenzene may decompose to benzene and toluene and also may react with hydrogen to form toluene and methane: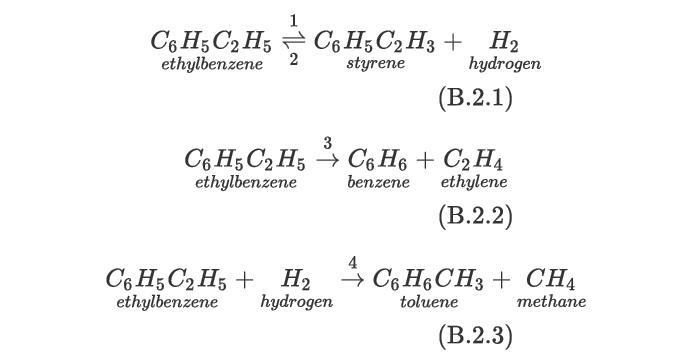
This process is presented in Appendix B as Project B.3 From the information given in Appendix B, determine the following:
1. The single-pass conversion of ethylbenzene
2. The overall conversion of ethylbenzene
3. The yield of styrene
Suggest one strategy to increase the yield of styrene, and sketch any changes to the PFD that this strategy would require.
Step by Step Answer:
Related Book For 

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting
Question Posted:




