The pressure of a fixed amount of compressed nitrogen gas in a cylinder is given, in atmospheres,
Question:
The pressure of a fixed amount of compressed nitrogen gas in a cylinder is given, in atmospheres, by
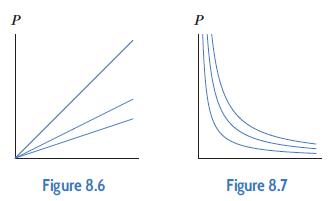
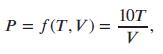 where T is the temperature of the gas, in Kelvin, and V is the volume of the cylinder, in liters. Figures 8.6 and 8.7 give cross sections of the function f.
where T is the temperature of the gas, in Kelvin, and V is the volume of the cylinder, in liters. Figures 8.6 and 8.7 give cross sections of the function f.
(a) Which figure shows cross-sections of f with T fixed? What does the shape of the cross-sections tell you about the pressure?
(b) Which figure shows cross-sections of f with V fixed? What does the shape of the cross-sections tell you about the pressure?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Applied Calculus
ISBN: 9781119275565
6th Edition
Authors: Deborah Hughes Hallett, Patti Frazer Lock, Andrew M. Gleason, Daniel E. Flath, Sheldon P. Gordon, David O. Lomen, David Lovelock, William G. McCallum, Brad G. Osgood, Andrew Pasquale
Question Posted:





