Question: a. Determine the gage pressure at point A in Fig. 3.36. b. If the barometric pressure is 737 mm of mercury, express the pressure at
a. Determine the gage pressure at point A in Fig. 3.36.
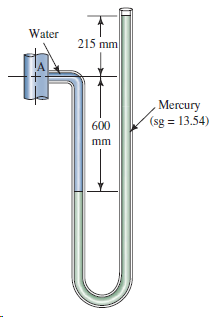
b. If the barometric pressure is 737 mm of mercury, express the pressure at point A in kPa(abs).
Water 215 mm Mercury (sg = 13.54) 600 mm
Step by Step Solution
3.36 Rating (168 Votes )
There are 3 Steps involved in it
a b p atm m h 1354 981 737 ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
1822_6046ffa6c9e22_724193.xlsx
300 KBs Excel File


