Question: Consider the endothermic reactions given below. Let A denote the event that a reaction's final temperature is 271 K or less. Let B denote the
Consider the endothermic reactions given below. Let A denote the event that a reaction's final temperature is 271 K or less. Let B denote the event that the heat absorbed is above target. Are these events independent?
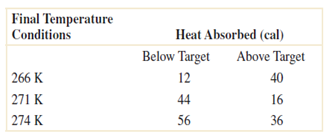
Final Temperature Conditions Heat Absorbed (cal) Below Target Above Target 266 K 12 40 271 K 44 16 274 K 56 36
Step by Step Solution
3.54 Rating (164 Votes )
There are 3 Steps involved in it
PA 112204 05490 PB 92204 ... View full answer

Get step-by-step solutions from verified subject matter experts


