According to Maxwells Distribution Law, in a gas of molecular mass m, the speed v of a
Question:
According to Maxwell’s Distribution Law, in a gas of molecular mass m, the speed v of a molecule in a gas at temperature T (kelvins) is a random variable with density
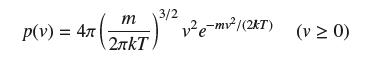
where k is Boltzmann’s constant. Show that the average molecular speed is equal to (8kT/πm)1/2. The average speed of oxygen molecules at room temperature is around 450 m/s.
Transcribed Image Text:
m 3/2 2лkТ p(v) = 4( v² e-mv²/(2T) (v≥ 0)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
The average speed vis given by So vf Thus Use Integration by Parts let ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Mathematics questions
-
Helium atoms have a mass of 4u and oxygen molecules have a mass of 32u, which is defined as an atomic mass unit (u=1.660540*10^-27 kg). Compare a gas of helium atoms to a gas of oxygen molecules. At...
-
(a) Explain why in a gas of N molecules, the number of molecules having speeds in the finite interval u to u + u is N = N f uu + uf(u) du. (b) If u is small, then f (u) is approximately constant over...
-
Molecules of a gas in a container are moving around at different speeds. Maxwell's speed distribution law gives the probability distribution P(v) as a function of temperature and speed: where M is...
-
When working as a tax professional, one issue that often arises is the need to provide difficult or disappointing guidance to a client. In the following example, determine the best course of action....
-
How can present value what-if analysis be enhanced by using software programs?
-
Consider the regression model fit to the gray range modulation data in Exercise 12-19. Use the useful range as the response. (a) Calculate 99% confidence intervals on each regression coefficient. (b)...
-
PRE P ARIN G CASH B U D G E T. Lucas and Emma Mendoza are preparing their 2018 cash budget. Help the Mendozas reconcile the following differences, giving reasons to support your answers a. Their only...
-
Spangler Company wrote off the following accounts receivable as uncollectible for the first year of its operations ending December 31, 2012: Customer Amount Will Boyette ..... $10,000 Stan Frey...
-
A cost is considered direct if it can be traced to a particular cost object in a cost effective way which means it can be A. possibly traced accurately with an investment in hardware and software. B....
-
The trough in Figure 18 is filled with corn syrup, whose weight density is 90 lb/ft 3 . Calculate the force on the front side of the trough. h b Buch. a
-
A square plate of side 3 m is submerged in water at an incline of 30 with the horizontal. Calculate the fluid force on one side of the plate if the top edge of the plate lies at a depth of 6 m.
-
A large flat horizontal sheet of charge has a charge per unit area of 9.00 %C/m2. Find the electric field just above the middle of the sheet.
-
Complete the exercises on the following website. Remember to type your answers in word or excel, screenshot, or phone pic as your work. The site does not save your answers. Upload your work on...
-
There are many management theories that are utilized in organizations. These theories were developed by scholars in the management discipline. One individual was responsible for identifying the major...
-
An increase in the price and a decrease of the quantity of Paclitaxel (an anti-cancer drug) could be caused by which of the following? Select one: O a. an increase in the number of people being...
-
At December 31, 2023, Cord Company's plant asset and accumulated depreciation and amortization accounts had balances as follows: Category Land Land improvements Buildings Equipment Automobiles and...
-
Assume that the following table represents the sales figures for the five largest firms in the industry. Compute the HHI for the industry (assuming the industry contains just these five firms). Sales...
-
Only 15% of 2,4-pentanedione exists as the enol tautomer in water, but 92% exists as the enol tautomer in hexane. Explain why this is so.
-
We all experience emotions, but some people disguise their true feelings better than others. Do you think this is a helpful or harmful thing to do? Under what conditions do you think it would be most...
-
Determine whether the following statements are true and give an explanation or counterexample. a. sin (a + b) = sin a + sin b. b. The equation cos = 2 has multiple real solutions. c. The equation...
-
Given the following information about one trigonometric function, evaluate the other five functions. sin = - 4/5 and < < 3/2
-
Given the following information about one trigonometric function, evaluate the other five functions. cos = 5/13 and 0 < < /2
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


