Question: 13. The table gives some information about the first five members of the carboxylic acid homologous series. (a) (1) Estimate the boiling point of pentanoic
13. The table gives some information about the first five members of the carboxylic acid homologous series.
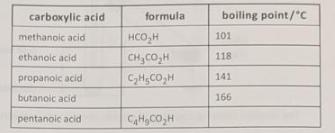
(a) (1) Estimate the boiling point of pentanoic acid. [1]
 (ii) Draw the structure of butanoic acid. Show all atoms and bonds. [1]
(ii) Draw the structure of butanoic acid. Show all atoms and bonds. [1]
(iii) Ethanoic acid reacts with sodium. Write an equation for this reaction. [1]
(b) Carboxylic acids react with alcohols to form esters.
(i) Name the ester formed when ethanoic acid reacts with ethanol. [1]
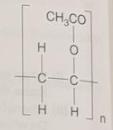
(ii) The diagram shows the repeat unit of poly(ethenyl ethanoate) Draw the structure of the monomer used to make poly(ethenyl ethanoate). [1]
(i) Calculate the empirical formula of X. [2]
(c) Carboxylic acid X contains 55.8% carbon, 7.0% hydrogen and 37.2% oxygen.
(ii) A molecule of carboxylic acid X contains four carbon atoms. What is its molecular formula? [1]
(iii) Carboxylic acid X is an unsaturated compound.
Give a test for an unsaturated compound. Include in your answer the test and the expected observation. [2]
carboxylic acid formula boiling point/"C methanoic acid 101 ethanoic acid CH, CO H 118 propanoic acid CHICOH 141 butanoic acid 166 pentanoic acid
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


