Calculate the energy that must be supplied to raise the temperature of 5.0 kg of water from
Question:
Calculate the energy that must be supplied to raise the temperature of 5.0 kg of water from 20 °C to 100 °C.
You will need to use data from Table 19.3 to answer these questions.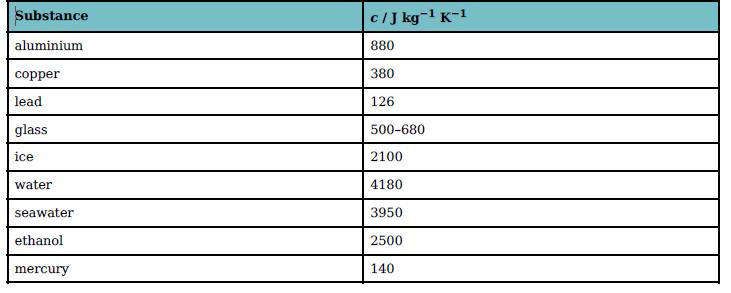
Transcribed Image Text:
Substance cIJ kg-1 K-1 aluminium 880 copper 380 lead 126 glass 500-680 ice 2100 water 4180 seawater 3950 ethanol 2500 mercury 140
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
we know the energy that must be supplied to raise th...View the full answer

Answered By

VAMSI TANGELLA
I have an experience of teaching about one year, i am good at solving questions and able to teach them in a way understandable by students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Cambridge International AS And A Level Physics Coursebook
ISBN: 9781108859035
3rd Edition
Authors: David Sang, Graham Jones, Gurinder Chadha, Richard Woodside
Question Posted:
Related Video
In this video, A mixture of methanol and air in a large polycarbonate bottle is ignited. The resulting rapid combustion reaction, often accompanied by a dramatic ‘whoosh’ sound and flames, demonstrates the large amount of chemical energy released in the combustion of alcohol
Students also viewed these Sciences questions
-
The heat transfer necessary to raise the temperature of a constant-volume closed system is given by Q = U. Using the SL model, compare the heat necessary to raise the temperature by 10oC for such a...
-
Estimate the energy required to raise the temperature of 5 kg (11.0 lbm) of the following materials from 20C to 150C (68F to 300F): aluminum, brass, aluminum oxide (alumina), and polypropylene.
-
The heat required to raise the temperature of m (kg) of a liquid from T 1 to T 2 at constant pressure is in high school and in first-year college physics courses, the formula is usually given as (a)...
-
Discuss any of the established brands of the business concerned with woolworths in Australia
-
Jie & Partners purchased a computer priced at $949.99, financing it by paying $75.12 on the date of purchase, and signing a contract to pay equal monthly payments over the next 15 months. If the...
-
Explain the effect on capacity reallocations of advance sales data indicating mean demand of 55 rather than 60 during a slow travel week for business class, using the information in Figure, Table,...
-
Using a spreadsheet for Problem 23, determine how Ninas ability to negotiate a lower rent at location 3, thereby raising its ranking to good, will affect the LO8 overall rankings of the four...
-
Pineapple Motor Company manufactures two types of specialty electric motors, a commercial motor and a residential motor, through two production departments, Assembly and Testing. Presently, the...
-
discuss the major differences between consumer and commercial loans.
-
Topmo Corporation has estimated it will have Factory Overhead cost of $1.2 Million, Direct Materials will be $12.4 Million, Indirect Labor will be $500,000 and Direct Labor Cost will be $3,920,000...
-
When a thermocouple has one junction in melting ice and the other junction in boiling water it produces an e.m.f. of 63 V. a. What e.m.f. would be produced if the second junction was also placed in...
-
a. The first law of thermodynamics can be represented by the expression: U = q + W. State what is meant by all the symbols in this expression. b. Figure 19.18 shows a fixed mass of gas that undergoes...
-
The hydraulic cylinder BC exerts on member AB a force P directed along line BC. Knowing that P must have a 600-N component perpendicular to member AB, determine (a) The magnitude of the force P, (b)...
-
2. (12 points) A researcher hypothesizes that watching Comedy Central reduces anger in prison inmates. A sample of 8 inmates is administered the State Anger/Hostility Scale, their mean score is 5.0...
-
6. A car manufacturer estimates that the cost of production of x cars of a certain model is C(x) = -20x + 0.01 x 2 - 800. How many cars should be produced for a minimum cost? [Please justify your...
-
Latania is a 34-year-old female with a series of volatile interpersonal relationships. She expresses extreme jealousy when her friends spend time with other friends, and she can't maintain romantic...
-
psychological models or psychological theories. Basically these are different ways to think about human behaviorwhat causes behavior and how best to adapt to mental illness or adjustment problems....
-
Product Development: Identify which sports team you are representing and what product you would recommend for the team license. Why did you choose this product? What target audience will purchase it
-
A taxpayer presented her tax return preparer, Dev Powell, with documentation supporting income she had earned as an independent contractor. Although Powell knew that the taxpayers income should be...
-
Juarez worked for Westarz Homes at construction sites for five years. Bever was a superintendent at construction sites, supervising subcontractors and moving trash from sites to landfills. He...
-
Calculate K P at 600.K for the reaction N 2 O 4 (l) 2NO 2 (g) assuming that H o R is constant over the interval 298 725 K.
-
Consider the equilibrium CO(g) + H2O(g) ???? CO2(g) + H2(g). At 1150. K, the composition of the reaction mixture is a. Calculate K P and ÎG o R at 1150. K. b. Given the answer to part (a), use...
-
When 2,4-dibromo-3-methyltoluene is treated with bromine in the presence of iron (Fe), a compound with molecular formula C8H7Br3 is obtained. Identify the structure of this product.
-
FINANCIAL STATEMENT ANALYSIS INSTRUCTIONS 1. PREPARE RATIO ANALYSIS REPORT ( word file) Format 1. Introduction 2. Importance of Financial Statements 3. Importance of Financial statement analysis and...
-
Let us assume that Europe is in recession, China's economy is slowing down, and the US economy is growing at 1-2%. Use these assumptions to invest in 4 ETFs (electronically traded funds). The 4 ETFs...
-
A section 83(b) election creates ordinary income at the time of the grant. Ture or False

Study smarter with the SolutionInn App


