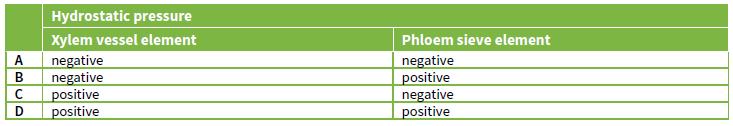
Which of the following rows correctly describes the hydrostatic pressure of the two types of elements? Hydrostatic
Question:
Which of the following rows correctly describes the hydrostatic pressure of the two types of elements?
Transcribed Image Text:
Hydrostatic pressure Xylem vessel element A negative B negative C positive D positive Phloem sieve element negative positive negative positive ים>
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
Answer B The movement of water from xylem vessels will low...View the full answer

Answered By

Jaseena vafa
Hands-on experience at the graduation and post-graduation level education facility
Familiarity working at a College and University
department
Outstanding ability to handle syllabus at the
undergraduate and graduate curriculum
Remarkable ability to teach, inspire and develop young
people
Excellent written and oral communication skills
Strong organizational skills and proficiency with MS
office tools.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Cambridge International AS And A Level Biology
ISBN: 9781107636828
4th Edition
Authors: Mary Jones, Richard Fosbery, Jennifer Gregory, Dennis Taylor
Question Posted:
Students also viewed these Sciences questions
-
Which of the following statements correctly describes an advantage that can be attributed to a Registered Education Savings Plan? A. Contributions to the plan can be deducted by the contributor. B....
-
1. The cantilever beam ab is subjected to the set of loads shown. It is stated thart-200 CPL I=3e8 mm and L=5 m. What is the value of Me that will make the deflection at wint Szer You may use any...
-
Which of the following describes recrystallization? (A) Diffusion dependent with a change in phase composition (B) Diffusionless (C) Diffusion dependent with no change in phase composition (D) All of...
-
JDBC applications are made of two models: two-tier and three-tier model. (True/False)
-
Indium compounds give a blue-violet flame test. The atomic emission responsible for this blue-violet color has a wavelength of 451 nm. Obtain the energy of a single photon of this wavelength.
-
Under the fixed exchange rate system, what was the currency against which all other currency values were defined? Why?
-
e Will you run your blog on an internal Emerson site or LD is je contract with an outside hosting service?
-
Information from Greg Companys balance sheet follows: Current assets: Cash ....................$ 2,100,000 Marketable securities ............. 7,200,000 Accounts receivable ............... 50,500,000...
-
You are analyzing the cost of debt for a firm. You know that the firms 14-year maturity, 8.6 percent coupon bonds are selling at a price of $1,016.29. The bonds pay interest semiannually. If these...
-
The homogeneous circular cylinder of mass m and radius R carries a slender rod of mass m/2 attached to it as shown. If the cylinder rolls on the surface without slipping with a velocity v O of its...
-
If sucrose is actively loaded into a sieve tube, which combination of changes takes place in the sieve tube? Solute potential decreases (becomes more negative) decreases (becomes more negative)...
-
The diagram shows the effect of light intensity on the rate of transpiration from the upper and lower epidermis of a leaf. Other environmental factors were kept constant. What could explain the...
-
Majesty Company uses target costing to ensure that its products are profitable. Assume Majesty is planning to introduce a new product with the following estimates: Required: 1. Compute the target...
-
Use the following information for questions 1 and 2. Caterpillar Financial Services Corp. (a subsidiary of Caterpillar) and Sterling Construction sign a lease agreement dated January 1, 2020, that...
-
Identifying Binomial Distributions. Determine whether the given procedure results in a binomial distribution or a distribution that can be treated as binomial (by applying the 5% guideline for...
-
Case 6: TOMS Shoes in 2016: An Ongoing Dedication to Social Responsibility, by Margaret A. Peteraf, Sean Zhand, and Meghan L. Cooney (page C-57) Read the case and then respond to the case questions...
-
Quatro Co. issues bonds dated January 1, 2019, with a par value of $740,000. The bonds' annual contract rate is 13%, and interest is paid semiannually on June 30 and December 31. The bonds mature in...
-
Wildcat Mining wants to know the appropriate discount rate to use in their capital budgeting decision making process. Based on the following data, what is the weighted average cost of capital the CFO...
-
Match each of the following graphs to the correct cost behavior. Cost Behavior1. Variable cost per unit.2. Total variable cost.3. Fixed cost per unit.4. Total fixed cost.5. Total mixed cost. Activity...
-
Design a circuit which negative the content of any register and store it in the same register.
-
Propose a structure for a compound with molecular formula C 4 H 8 O that exhibits the following 13 C NMR and FTIR spectra. Carbon NMR 67.7- 25.4- 80 30 20 100 90 70 60 40 10 Chemical shift (ppm) 100...
-
Propose a structure for a compound with molecular formula C 4 H 10 O that exhibits the following 1 H NMR spectrum. Proton NMR 2 3.0 4.0 2.5 Chemical shift (ppm) 2.0 3.5 1.5 1.0
-
Predict the product of the following reaction. M 1) LIAID, 2) H20 Me
-
Question 7 of 7 0/14 W PIERDERY Current Attempt in Progress Your answer is incorrect Buffalo Corporation adopted the dollar value LIFO retail inventory method on January 1, 2019. At that time the...
-
Cost of debt with fees . Kenny Enterprises will issue a bond with a par value of $1,000, a maturity of twenty years, and a coupon rate of 9.9% with semiannual payments, and will use an investment...
-
Assume that an investment of $100,000 is expected to grow during the next year by 8% with SD 20%, and that the return is normally distributed. Whats the 5% VaR for the investment? A. $24,898 B....

Study smarter with the SolutionInn App


