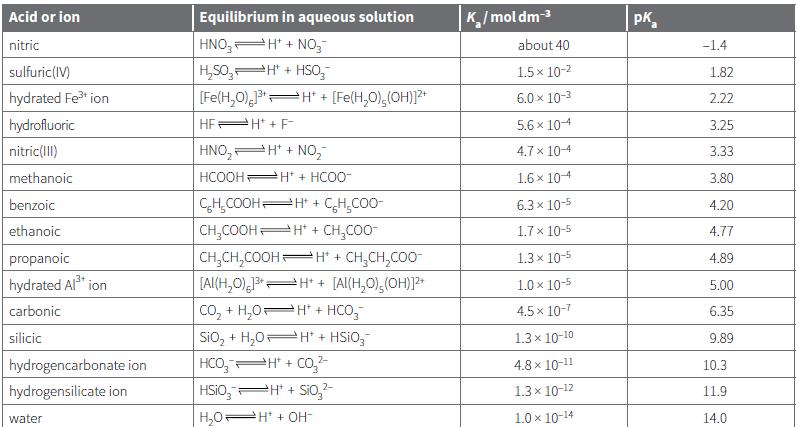
Use the data from Table 21.2 to work out the pH values of the following solutions: a.
Question:
Use the data from Table 21.2 to work out the pH values of the following solutions:
a. 0.0200 mol dm–3 aqueous benzoic acid
b. 0.0100 mol dm–3 hydrated aluminium ions
c. 0.100 mol dm–3 aqueous methanoic acid.
Transcribed Image Text:
Acid or ion Equilibrium in aqueous solution K/moldm-3 pK, nitric HNO, H' + NO,- about 40 -1.4 H,S0, H" + HSo, [Fe(H,0), H" + sulfuric(IV) 1.5 x 10-2 1.82 hydrated Fe ion [Fe(H,O),(OH))?* 6.0 x 10-3 2.22 hydrofluoric HEPH + F- 5.6 x 104 3.25 nitric(1II) HNO, H* + NO, 4.7 x 104 3.33 methanoic HCOOH Ht + HCO0- 1.6 x 104 3.80 CH,COOHH* + CH,CO0- CH,COOH H* + CH,CO0- benzoic 6.3 x 10-5 4.20 ethanoic 1.7 x 10-5 4.77 propanoic CH,CH,COOH H* + CH,CH,COO- 1.3 x 10-5 4.89 [Al(H,0) H* + [Al(H,0),(OH)]? Co, + H,0H* + HCO,- Sio, + H,0 H* + HSIO,- HCO, H + Co,2- hydrated Al* ion 1.0 x 10-5 5.00 carbonic 4.5 x 10-7 6.35 silicic 1.3 x 10-10 9.89 hydrogencarbonate ion 4.8 x 10-11 10.3 hydrogensilicate ion HSIO,PH + Sio,- 1.3x 10-12 11.9 water H,0H* + OH- 1.0 x 10-14 14.0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 91% (12 reviews)
a The concentration of aqueous benzoic acid is 002 moldm3 ka Value of acid is 631...View the full answer

Answered By

Samee Ullah
Algebra, Linear algebra, calculus, accounting, marketing, statistics, programming, real estate, writing, human resource management, business communication, Engineering: civil, chemical, electrical, mechanical, aerospace, building
Linguistics: sociolinguistics, applied linguistics, music, social sciences, biology, chemistry: all types, Thermodynamics, mechanics, modern physics, quantum physics, metaphysics, biology.
Feel free to contact us for all these subjects,; for quality, and best responses. Thankyou
0.00
0 Reviews
10+ Question Solved
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:
Students also viewed these Sciences questions
-
Use the data from The Wall Street Journal in Figure to verify the trin ratio for the NYSE. Is the trin ratio bullish orbearish? Trading Diary: Volume, Advancers, Decliners Markets Diary Issues...
-
Use the data from Exercise 15.
-
Use the data from The Financial Post in Figure 10.8 to construct the trin ratio for the TSX. Is the trin ratio bullish or bearish? Figure 10.8 Market breadth. Market breadth TRADING (1000s) ASWANCE...
-
Energy of the emitted photon when an L-electron drops into the k-state in copper (z = 29) is -1 use R=109737 cm, cm =1.23910eV] 7994.6 eV 1094.6 eV 1293.6 eV 1097.3 eV
-
Sketch the areas under the standard normal curve over the indicated intervals and find the specified areas. To the left of z = 0.47
-
Nite-Site, Inc., manufactures image intensification devices used in products such as night-vision goggles and aviators night-vision imaging systems. The primary customer for these products is the...
-
Do managers have a responsibility to ensure that conflict is functional or to eliminate dysfunctional conflict?p. 408
-
Mainland Supply Company recently changed its system of internal control over cash disbursements. The system includes the following features. Instead of being unnumbered and manually prepared, all...
-
On January 1, a company issues bonds dated January 1 with a par value of $220,000. The bonds mature in 5 years. The contract rate is 9%, and interest is paid semiannually on June 30 and December 31....
-
The mean per capita daily water consumption in a village in Bangladesh is about 83 liters per person and the standard deviation is about 11.9 liters per person. Random samples of size 50 are drawn...
-
A buffer solution consists of 6.00 g of ethanoic acid (CH 3 COOH) and 12.3 g of sodium ethanoate (CH 3 COONa) in 200 cm 3 of aqueous solution. (A r values: H = 1.0, C = 12.0, O = 16.0, Na = 23.0; Ka...
-
A saturated solution of copper(I) sulfide, Cu 2 S, contains 1.91 10 12 g of Cu 2 S dissolved in 1 dm 3 of water. (A r values: Cu = 63.5, S = 32.1) a. Write an equilibrium expression for the...
-
Prist Co. had not provided a warranty on its products, but competitive pressures forced management to add this feature at the beginning of 2010. Based on analysis of customer complaints made over the...
-
Q. Probabilities of three teams A, B and C of winning the first prize of a business case competition are 3/9, 2/9, 3/9 respectively. These three teams are equally likely to win the prize. True False...
-
Univex is a calendar year, accrual basis retail business. Univex hold less than 20% of IBM stock. Its financial statements provide the following information for the year: Revenues from sales of goods...
-
Consider the unadjusted trial balance of Fabuloso, Inc. at December 31, 2023, and the related month-end adjustment data. (Click the icon to view the month-end adjustment data.) Requirements 1....
-
5.3 BEP Example Bill Braddock is considering opening a Fast 'n Clean Car Service Center. He estimates that the following costs will be incurred during his first year of operations: Rent $9,200,...
-
The following is the text for an opinion on internal control for W Company, an issuer. Some words or phrases have been replaced with numbers (e.g., [1], [2], etc.). Select from the option list...
-
Kerianne paints landscapes, and in late 2021 placed four paintings with a retail price of $250 each in the Holmstrom Gallery. Keriannes arrangement with Holmstrom is that Holmstrom will earn a 20%...
-
United Business Forms capital structure is as follows: Debt ............................................ 35% Preferred stock ........................... 15 Common equity .......................... 50...
-
Identify the reactants that you would use to make each of the following enamines: (a) (b) (c) -N
-
Predict the product of the two-step procedure below, and draw a mechanism for its formation: 1) [H1, -N-NH2 -H2 2) / -0, heat
-
Propose a plausible mechanism for each of the following hydrolysis reactions: (a) (b) (c) (d) EtO OEt * * + 2 ELOH (b) N. * .N'
-
4) Read the following case carefully and answer the given questions. You have been the finance director of a clothing retailer for ten years. The companys year end is 31st December 2019, and you are...
-
all of the other problems here on chegg don't describe right on how they god the answer. can you make it step by step math to show how you got what and from where and each number to get the answer...
-
D Required information The following Information applies to the questions displayed below.) Diego Company manufactures one product that is sold for $76 per unit in two geographic regions-the East and...

Study smarter with the SolutionInn App


