Question: Redo Problem 13.14 using Aspen Plus. Problem 13.14 Acetaldehyde is produced from ethanol by the following gas-phase reactions: The reactions are carried out at 540C
Redo Problem 13.14 using Aspen Plus.
Problem 13.14
Acetaldehyde is produced from ethanol by the following gas-phase reactions:
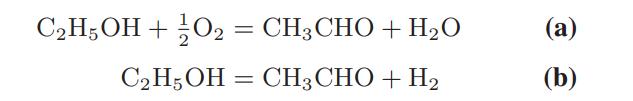
The reactions are carried out at 540°C and 1 bar pressure using a silver gauze catalyst and air as an oxidant. If 50% excess air [sufficient air that 50 percent more oxygen is present than is needed for all the ethanol to react by reaction (a)] is used, calculate the equilibrium composition of the reactor effluent.
CH5OH + O = CH3CHO + HO 02 C2H5OH CH3CHO + H = (a) (b)
Step by Step Solution
3.44 Rating (157 Votes )
There are 3 Steps involved in it
here is a stepbystep guide to solving Problem 1314 using Aspen Plus Create a new Aspen Plu... View full answer

Get step-by-step solutions from verified subject matter experts


