Question: Redo Problem 13.21 using Aspen Plus. Problem 13.21 Styrene can be hydrogenated to ethyl benzene at moderate conditions in both the liquid phase and the
Redo Problem 13.21 using Aspen Plus.
Problem 13.21
Styrene can be hydrogenated to ethyl benzene at moderate conditions in both the liquid phase and the gas phase. Calculate the equilibrium compositions in the vapor and liquid phases of hydrogen, styrene, and ethyl benzene at each of the following conditions:
a. 3-bar pressure and 25°C, with a starting mole ratio of hydrogen to styrene of 2 to 1
b. 3-bar pressure and 150°C, with a starting mole ratio of hydrogen to styrene of 2 to 1
Data:
Reaction stoichiometry:
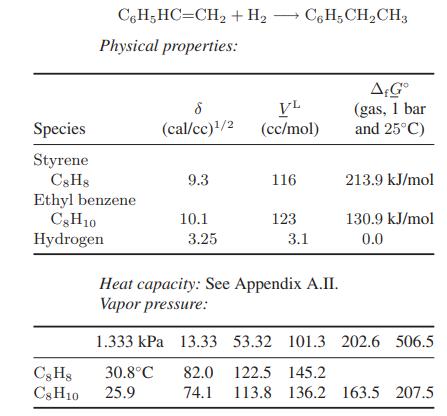
Appendix A.II.
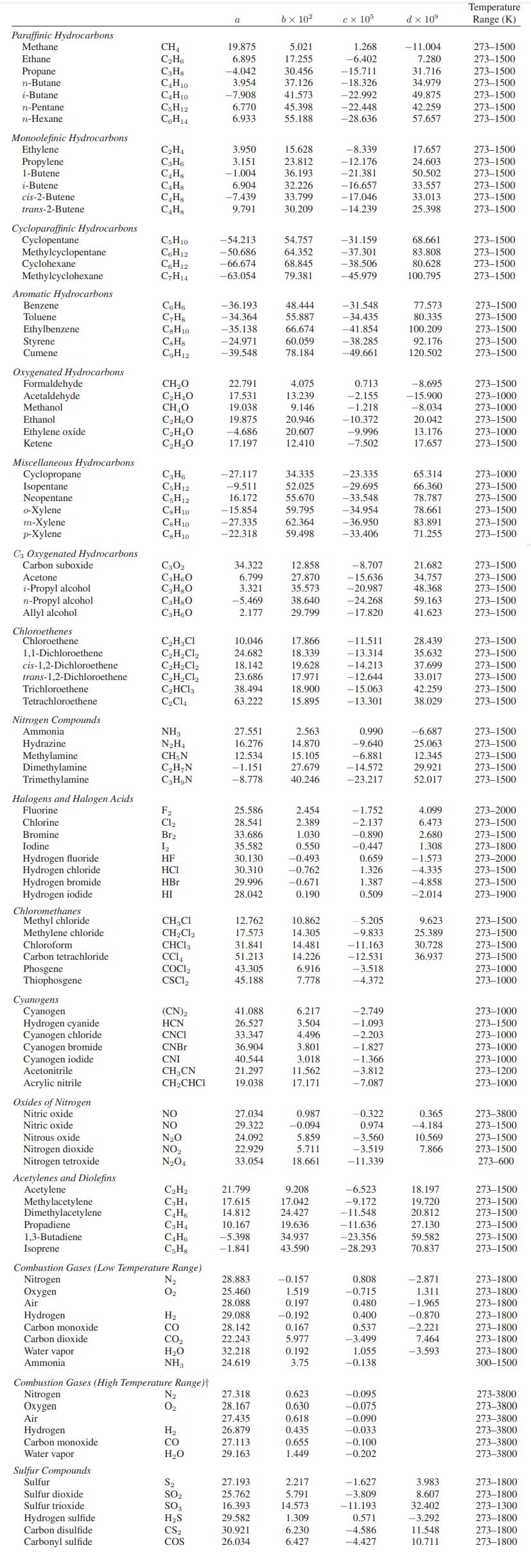
C6H5HC=CH + H C6H5 CHCH3 Physical properties: Species Styrene C8H8 Ethyl benzene C8H10 Hydrogen Cg Hs C8H10 8 VL (cal/cc)/2 (cc/mol) 9.3 30.8C 25.9 10.1 3.25 116 123 3.1 Heat capacity: See Appendix A.II. Vapor pressure: AfGo (gas, 1 bar and 25C) 213.9 kJ/mol 130.9 kJ/mol 0.0 1.333 kPa 13.33 53.32 101.3 202.6 506.5 82.0 122.5 145.2 74.1 113.8 136.2 163.5 207.5
Step by Step Solution
3.35 Rating (158 Votes )
There are 3 Steps involved in it
o solve Problem 1321 using Aspen Plus we need to determine the equilibrium compositions for the reaction C 6 H 5 C H C H 2 H 2 C 6 H 5 C H 2 C H 3 C6H5CHCH2 H2 rightarrow C6H5CH2CH3 C6H5CHCH2H2C6H5CH2... View full answer

Get step-by-step solutions from verified subject matter experts


