Question: Redo Problem 13.28 using Aspen Plus. Problem 13.28 A gas mixture containing equimolar quantities of carbon dioxide and hydrogen is to be reformed by passing
Redo Problem 13.28 using Aspen Plus.
Problem 13.28
A gas mixture containing equimolar quantities of carbon dioxide and hydrogen is to be “reformed” by passing it over a catalyst. The pressure in the reformer will be determined by the possibility of solid carbon deposition. Although a large number of reactions are possible, only the following are believed to occur:
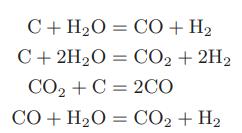
a. At temperatures between 600 and 1000 K, over what range of pressure will carbon deposit if each of the reactions is assumed to achieve equilibrium?
b. For this feed, what pressure should be maintained for exactly 30 percent of the carbon present in the feed to precipitate as solid carbon at each temperature between 600 and 1000 K?
C+H,O=CO+H, C+2HO = CO2 + 2H CO,+C =2CO CO+H2O = CO2+H2
Step by Step Solution
3.45 Rating (161 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


