Question: Repeat Problem 5.10 assuming that nitrogen is described by the Peng-Robinson equation of state. Problem 5.10 The Quick Fill bicycle tire filling system consists of
Repeat Problem 5.10 assuming that nitrogen is described by the Peng-Robinson equation of state.
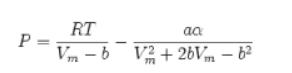
Problem 5.10
The “Quick Fill” bicycle tire filling system consists of a small (2 cm diameter, 6.5 cm long) cylinder filled with nitrogen to a pressure of 140 bar. Estimate the explosion equivalent of the gas contained in the cylinder in grams of TNT. Assume nitrogen is an ideal gas.
P = RT aa Vm-b V2+2bVm b
Step by Step Solution
3.46 Rating (156 Votes )
There are 3 Steps involved in it
To solve this problem we need to calculate the number of moles of nitrogen gas in the cylinder and t... View full answer

Get step-by-step solutions from verified subject matter experts


