(100 mathrm{kmol} / mathrm{h}) of an (8 mathrm{~mol} %) naphthalene (-92 mathrm{~mol} %) phenol mixture at (40^{circ}...
Question:
\(100 \mathrm{kmol} / \mathrm{h}\) of an \(8 \mathrm{~mol} \%\) naphthalene \(-92 \mathrm{~mol} \%\) phenol mixture at \(40^{\circ} \mathrm{C}\) is fed to a continuous crystallizer. The crystallizer operates at \(33^{\circ} \mathrm{C}\). Data are in Figure 18-1.
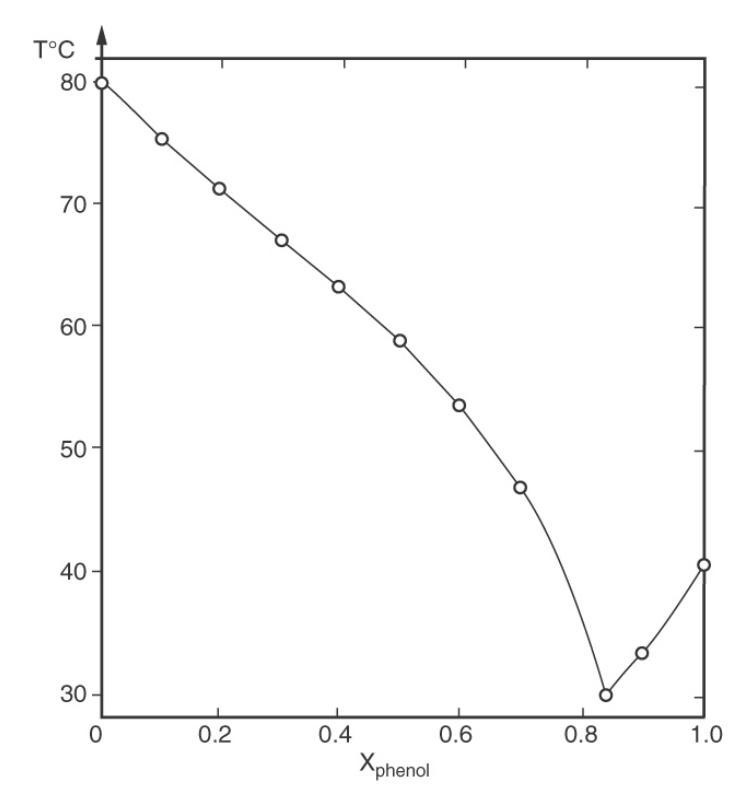
a. What is the purity of crystals?
b. What is the approximate concentration of the final melt?
c. How many \(\mathrm{kmol} / \mathrm{h}\) of crystals are harvested, and what is the flow rate, \(\mathrm{kmol} / \mathrm{h}\), of the mother liquor?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat
Question Posted:





