A fractional extraction system (Figure 13-5) is separating abietic acid from other acids. Solvent 1 , heptane,
Question:
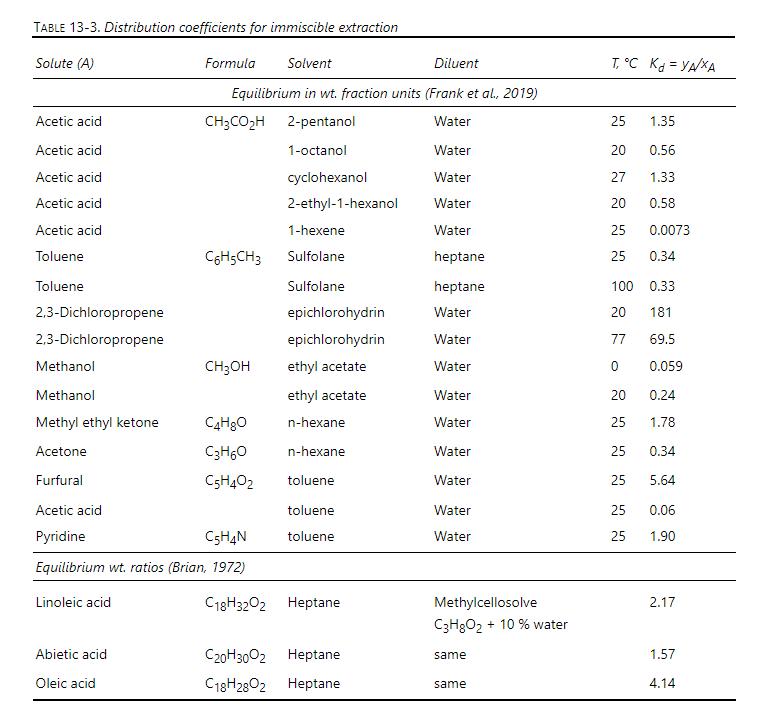
A fractional extraction system (Figure 13-5) is separating abietic acid from other acids. Solvent 1 , heptane, enters at \(\mathrm{E}-=1000 \mathrm{~kg} / \mathrm{h}\) and is pure. Solvent 2, methylcellosolve \(+10 \%\) water, is pure and has a flow rate of \(\mathrm{R}=2500 \mathrm{~kg} / \mathrm{h}\). Feed is \(5 \mathrm{wt} \%\) abietic acid in solvent 2 and flows at \(1 \mathrm{~kg} / \mathrm{h}\). There are only traces of other acids in the feed. We desire to recover \(95 \%\) of the abietic acid in the bottom raffinate stream. Feed is on stage 6. Assume solvents are completely immiscible, and system can be considered to be very dilute. Equilibrium data are given in Table 13-3. Find \(\mathrm{N}\) using a McCabe-Thiele diagram.
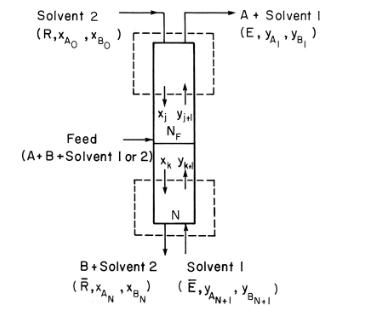
Table 13-3
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





