Question: A strong base resin is exchanging (mathrm{NO}_{3}{ }^{-})with (mathrm{Cl}^{-}). The resin capacity is (mathrm{c}_{mathrm{RT}}=1.25 mathrm{eq} / mathrm{L}, varepsilon_{mathrm{e}}) (=0.40), and total ion concentration is (mathrm{c}_{mathrm{T}}=0.85
A strong base resin is exchanging \(\mathrm{NO}_{3}{ }^{-}\)with \(\mathrm{Cl}^{-}\). The resin capacity is \(\mathrm{c}_{\mathrm{RT}}=1.25 \mathrm{eq} / \mathrm{L}, \varepsilon_{\mathrm{e}}\) \(=0.40\), and total ion concentration is \(\mathrm{c}_{\mathrm{T}}=0.85 \mathrm{eq} / \mathrm{L}\) throughout the process. The \(0.825 \mathrm{~m}\) long column is initially in \(\mathrm{NO}_{3}{ }^{-}\)form, and at \(\mathrm{t}=0\), a feed with \(\mathrm{x}_{\mathrm{NO} 3}=0.60\left(\mathrm{x}_{\mathrm{Cl}}=0.40\right)\) is fed at \(\mathrm{v}_{\text {super }}=18 \mathrm{~cm} / \mathrm{min}\). This feed continues for 20 minutes at which time a feed with \(\mathrm{x}_{\mathrm{NO} 3}=0\) enters (same velocity). Use ion movement theory to predict the \(\mathrm{NO}_{3}^{-}\)and \(\mathrm{Cl}^{-}\) outlet concentration profiles. Equilibrium data is in Table 20-5.
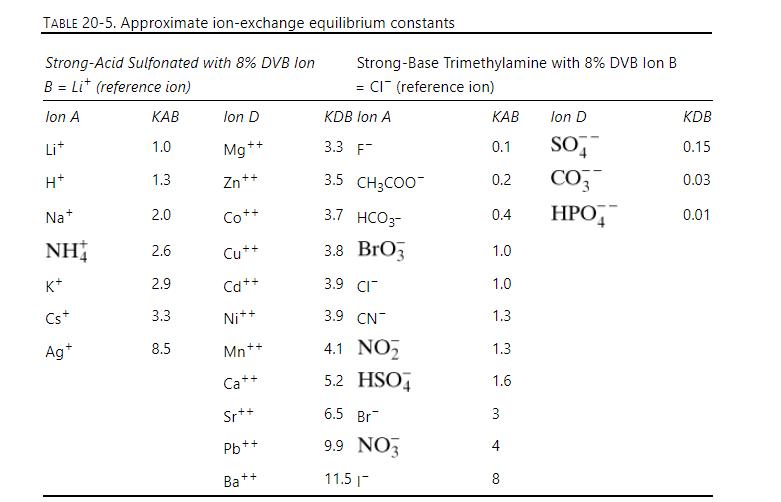
TABLE 20-5. Approximate ion-exchange equilibrium constants Strong-Acid Sulfonated with 8% DVB lon B=Li (reference ion) Strong-Base Trimethylamine with 8% DVB Ion B = CI (reference ion) Ion A KAB Ion D KDB Ion A KAB Ion D KDB Li+ 1.0 Mg++ 3.3 F 0.1 SO 0.15 H+ 1.3 Zn++ 3.5 CH3COO 0.2 CO3 0.03 Na+ 2.0 Co++ 3.7 HCO3- +0 0.4 0.01 NH 2.6 Cu++ 3.8 BrO3 1.0 K+ 2.9 Cd++ 3.9 CI 1.0 Cs+ 3.3 Ni++ 3.9 CN 1.3 Ag+ 8.5 Mn++ 4.1 NO Ca++ 5.2 HSO 36 1.3 1.6 Sr++ 6.5 Br 3 Pb++ 9.9 NO 4 Ba+ ++ 11.5|- 8
Step by Step Solution
3.38 Rating (167 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


