An aqueous mixture that is (30 mathrm{wt} % mathrm{Mn}left(mathrm{NO}_{3} ight)_{2}), initially at (20^{circ} mathrm{C}), is cooled slowly
Question:
An aqueous mixture that is \(30 \mathrm{wt} \% \mathrm{Mn}\left(\mathrm{NO}_{3}\right)_{2}\), initially at \(20^{\circ} \mathrm{C}\), is cooled slowly so that it is always at equilibrium. Data are in Figure 17-6.
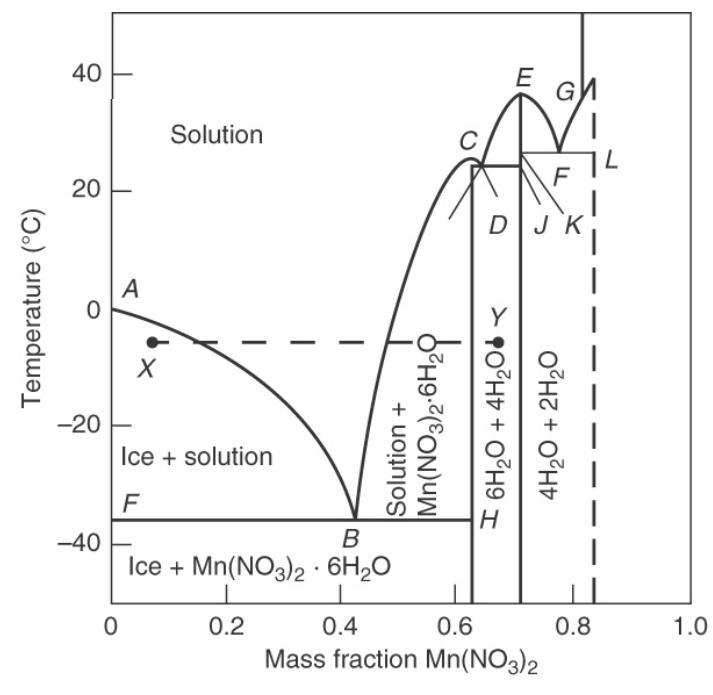
a. At \(0^{\circ} \mathrm{C}\) what phase(s) are present, and what is (are) their composition(s)?
b. At \(-20^{\circ} \mathrm{C}\) what phase(s) are present, and what is (are) their composition(s)?
c. At \(-20^{\circ} \mathrm{C}\) how many \(\mathrm{kg}\) per \(\mathrm{kg}\) of feed do we have of each phase present?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat
Question Posted:





