An extractive distillation system is separating ethanol from water using ethylene glycol as solvent. The makeup solvent
Question:
An extractive distillation system is separating ethanol from water using ethylene glycol as solvent. The makeup solvent is pure ethylene glycol. In Figure 8-14, ethanol is A product, and water is B product. The feed is 20.0 \(\mathrm{mol} \%\) ethanol, and the remainder is water with flow rate of \(100.0 \mathrm{kmol} / \mathrm{h}\). The ethanol product (column 1 distillate) is \(99.70 \mathrm{~mol} \%\) ethanol, 0.02 \(\mathrm{mol} \%\) ethylene glycol, and the remainder is water. The water product (column 2 distillate) is \(99.90 \mathrm{~mol} \%\) water, \(0.035 \mathrm{~mol} \%\) ethylene glycol, and the remainder is ethanol.
Figure 8-14
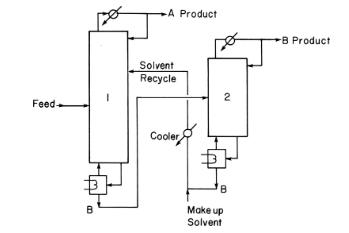
a. Find the flow rates of the makeup solvent and of the ethanol and water products.
b. Why would a separation flowsheet that uses a normal distillation column to preconcentrate the feed be chosen instead of this separation?
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





