Benzene-toluene equilibrium is often approximated as (alpha_{mathrm{BT}}=2.34). Generate the (mathrm{y}-mathrm{x}) diagram for this relative volatility. Compare your
Question:
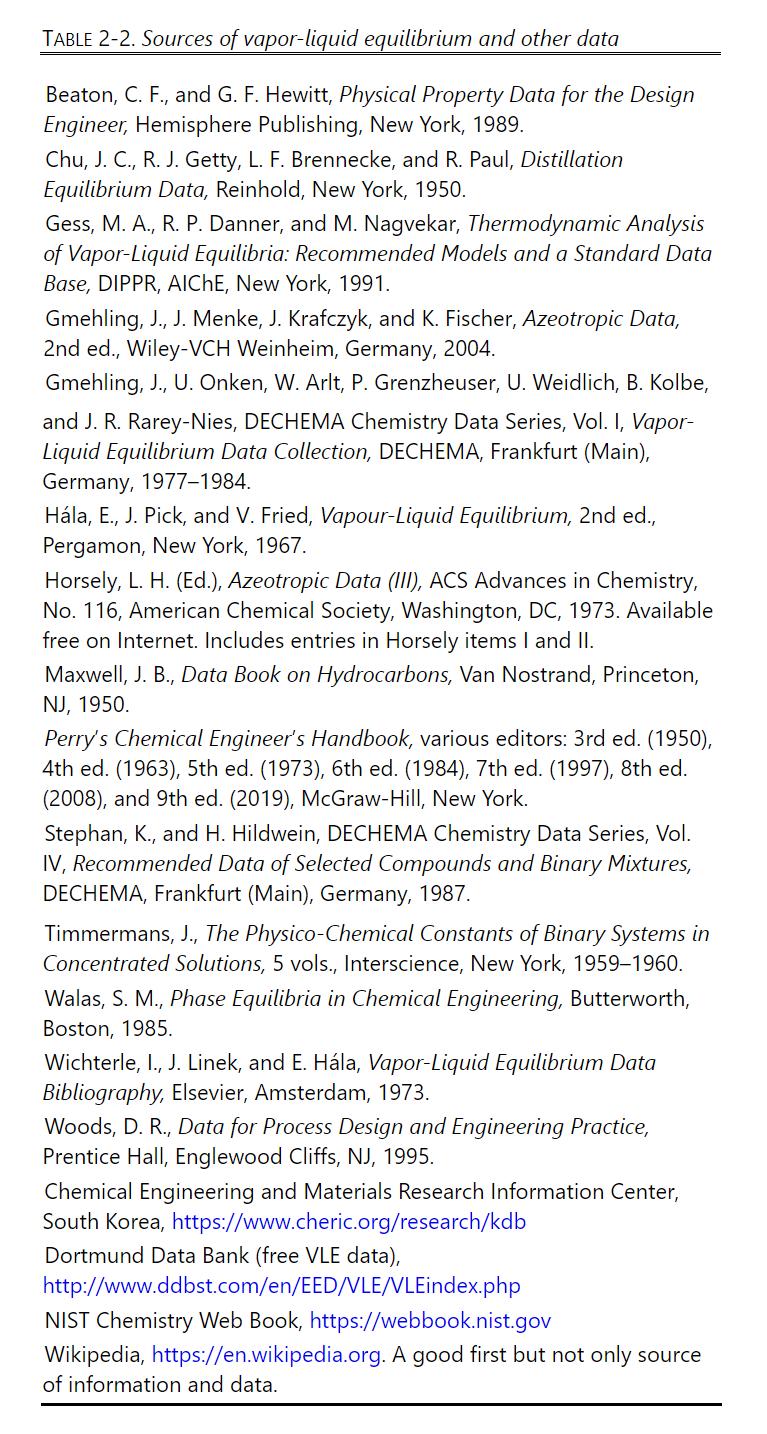
Benzene-toluene equilibrium is often approximated as \(\alpha_{\mathrm{BT}}=2.34\). Generate the \(\mathrm{y}-\mathrm{x}\) diagram for this relative volatility. Compare your results with data in the literature (see references in Table 2-2). Also, generate the equilibrium data using Raoult's law, and compare your results to these.
Transcribed Image Text:
TABLE 2-2. Sources of vapor-liquid equilibrium and other data Beaton, C. F., and G. F. Hewitt, Physical Property Data for the Design Engineer, Hemisphere Publishing, New York, 1989. Chu, J. C., R. J. Getty, L. F. Brennecke, and R. Paul, Distillation Equilibrium Data, Reinhold, New York, 1950. Gess, M. A., R. P. Danner, and M. Nagvekar, Thermodynamic Analysis of Vapor-Liquid Equilibria: Recommended Models and a Standard Data Base, DIPPR, AIChE, New York, 1991. Gmehling, J., J. Menke, J. Krafczyk, and K. Fischer, Azeotropic Data, 2nd ed., Wiley-VCH Weinheim, Germany, 2004. Gmehling, J., U. Onken, W. Arlt, P. Grenzheuser, U. Weidlich, B. Kolbe, and J. R. Rarey-Nies, DECHEMA Chemistry Data Series, Vol. I, Vapor- Liquid Equilibrium Data Collection, DECHEMA, Frankfurt (Main), Germany, 1977-1984. Hla, E., J. Pick, and V. Fried, Vapour-Liquid Equilibrium, 2nd ed., Pergamon, New York, 1967. Horsely, L. H. (Ed.), Azeotropic Data (III), ACS Advances in Chemistry, No. 116, American Chemical Society, Washington, DC, 1973. Available free on Internet. Includes entries in Horsely items I and II. Maxwell, J. B., Data Book on Hydrocarbons, Van Nostrand, Princeton, NJ, 1950. Perry's Chemical Engineer's Handbook, various editors: 3rd ed. (1950), 4th ed. (1963), 5th ed. (1973), 6th ed. (1984), 7th ed. (1997), 8th ed. (2008), and 9th ed. (2019), McGraw-Hill, New York. Stephan, K., and H. Hildwein, DECHEMA Chemistry Data Series, Vol. IV, Recommended Data of Selected Compounds and Binary Mixtures, DECHEMA, Frankfurt (Main), Germany, 1987. Timmermans, J., The Physico-Chemical Constants of Binary Systems in Concentrated Solutions, 5 vols., Interscience, New York, 1959-1960. Walas, S. M., Phase Equilibria in Chemical Engineering, Butterworth, Boston, 1985. Wichterle, I., J. Linek, and E. Hla, Vapor-Liquid Equilibrium Data Bibliography, Elsevier, Amsterdam, 1973. Woods, D. R., Data for Process Design and Engineering Practice, Prentice Hall, Englewood Cliffs, NJ, 1995. Chemical Engineering and Materials Research Information Center, South Korea, https://www.cheric.org/research/kdb Dortmund Data Bank (free VLE data), http://www.ddbst.com/en/EED/VLE/VLEindex.php NIST Chemistry Web Book, https://webbook.nist.gov Wikipedia, https://en.wikipedia.org. A good first but not only source of information and data.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat
Question Posted:
Students also viewed these Engineering questions
-
In Problem 13.7, you should have simulated the reactor as a stoichiometric reactor with 75% per pass conversion. In order to estimate the volume of the reactor, it is necessary to have kinetics...
-
The Franklin Tire Company is interested in demonstrating the durability of its steel-belted radial tires. To do this, the managers have decided to put four tires on 100 different sport utility...
-
The new line character is utilized solely as the last person in each message. On association with the server, a client can possibly (I) question the situation with a client by sending the client's...
-
What were the worst mistakes made by Globestelle during the production development process ? CASE 15 Case date 2001 The Reltex Project Nigel Slack Globestelle is the world's largest precision...
-
Verify the expressions in (12.2.7). by'+ ab 1+82 (12.2.7) z,
-
In the model, buyers know the utility function of sellers, but do not know anything about the general quality of cars for sale. Indicate whether the statement is true or false, and justify your...
-
How can companies use the 4Ps to strengthen their position in an international market?
-
The following selected transactions were taken from the records of Shaw Company for the first year of its operations ending December 31, 2008: Jan. 31. Wrote off account of B. Roberts, $2,400. Mar....
-
Xavier is keen to help different organization. So, he makes two payments during the year ended 30 June 2019. $600 to the St. Vincent de Paul Society (a registered charity) to attend charity dinner...
-
Ethylene glycol and water are flash distilled in a cascade of three drums connected as shown in the figure. All drums operate at 228 mm Hg. Feed is 40 mol% water. One-third of the feed is vaporized...
-
What authority should internal auditors be accorded in terms of corrective action?
-
Agranoff Corporation purchased two zero-coupon bonds on January 1, 20X1. The first bond was issued by Lilah Company. It had a face amount of $100,000 and was scheduled to mature on December 31, 20X5....
-
Claim: Fewer than 8.2% of homes have only a landline telephone and no wireless phone. Sample data: A survey by the National Center for Health Statistics showed that among 13,215 homes 5.78% had...
-
Among 450 randomly selected drivers in the 16 - 18 age bracket, 374 were in a car crash in the last year. If a driver in that age bracket is randomly selected, what is the approximate probability...
-
Construct a 90% confidence interval for the population standard deviation o at Bank A. Bank A 6.4 6.6 6.7 6.8 7.1 7.2 7.6 7.8 7.8 7.8
-
In 2002, after the accounting deceptions of the management of many multi-million dollar corporations (with Enron being the benchmark name of that time period), the Security and Exchange Commission...
-
1.Deduce the structure of a compound with molecular formula CsH100 that exhibits the following IR, H NMR, and 13C NMR spectra. Data from the mass spectrum are also provided. Mass Spec. Data relative...
-
The balances of the ledger accounts of Pelango Furniture as of December 31, the end of its fiscal year, are as follows: Cash ....................... $ 12,482 Accounts Receivable ...................
-
DC has unused FTC carryover from 2017 in the separate category for GC income as the result of income generated by a foreign branch. The income was foreign source general category income. In 2018 the...
-
Adirondack Marketing Inc. manufactures two products, A and & Presently, the company uses a single plantwide factory overhead rate for allocating overhead to products, However, management is...
-
9. value: 10.00 points < Question 9 (of 10) P3-18 Using the Du Pont Identity [LO3] Y3K, Inc., has sales of $4,500, total assets of $3,460, and a debt-equity ratio of 1.40. If its return on equity is...
-
Consider the simple income statement model below. The company would like donate 15% of its net income to charity. How much will they contribute? Contribution Rate 15% Income Tax Rate 40% Circular...

Study smarter with the SolutionInn App


