Design a stripping column to remove carbon dioxide from water by heating the water and passing it
Question:
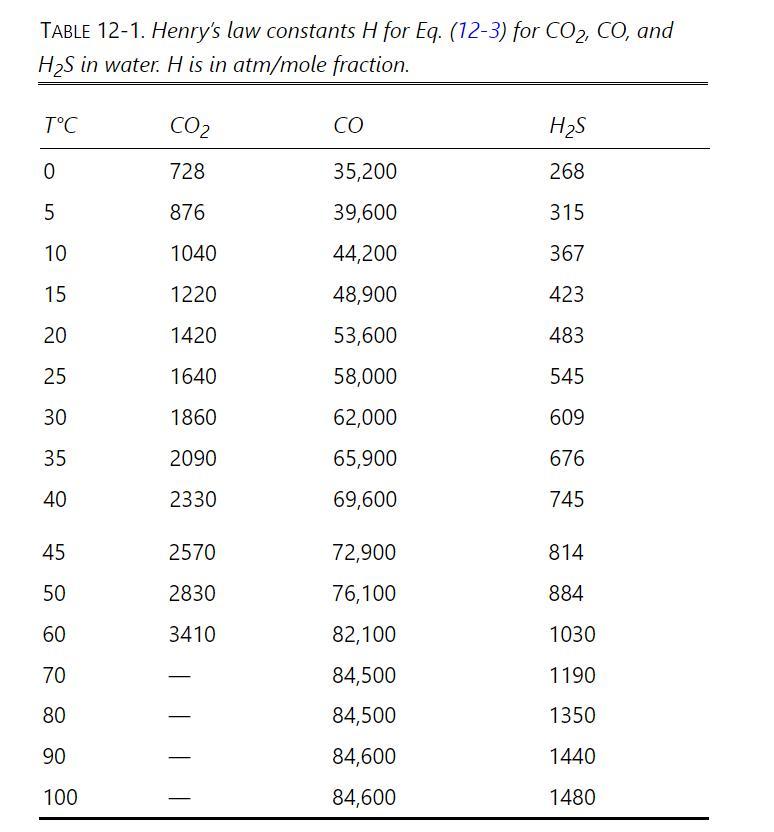
Design a stripping column to remove carbon dioxide from water by heating the water and passing it countercurrently to a nitrogen stream in a staged stripper. Operation is isothermal and isobaric at \(60.0^{\circ} \mathrm{C}\) and \(1.0 \mathrm{~atm}\) pressure. Feed water contains \(9.2 \times 10^{-6}\) mole fraction \(\mathrm{CO}_{2}\) and flows at \(100,000 \mathrm{lb} / \mathrm{h}\). Entering nitrogen is pure and flows at \(2500.0 \mathrm{ft}^{3} / \mathrm{h}\). Nitrogen is at \(1.0 \mathrm{~atm}\) and \(60.0^{\circ} \mathrm{C}\). Outlet water concentration is \(2.0 \times 10^{-7} \mathrm{~mole}\) fraction \(\mathrm{CO}_{2}\). Ignore nitrogen solubility in water, and ignore water volatility. Equilibrium data are in Table 12-1. Use a Murphree vapor efficiency of \(40.0 \%\). Find the outlet vapor composition and the number of real stages needed.
Table 12-1
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





