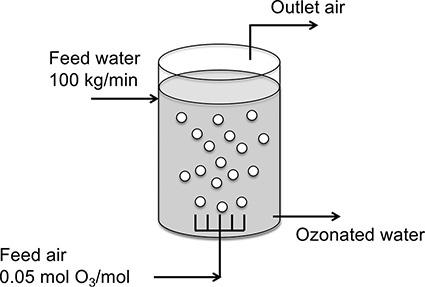
Ozonization of water can be used to control odor and for disinfection. In the simple ozonizer sketched
Question:
Ozonization of water can be used to control odor and for disinfection.
In the simple ozonizer sketched in Fig. P.8.1, air containing 5.0 mol % O3 is bubbled through water at 20°C flowing at the rate of 100 kg/min. The absolute pressure in the ozonizer is 1.2 atm. The solubility of pure O3 gas in water at 1 atm and 20°C is 0.57 g/L.
(a) What is the maximum concentration of O3 in water that can be obtained for these conditions?
Express your result in g/L and as a mole fraction.
(b) In the actual operation, the mole fraction of O3 in the ozonated water is 4.5 × 10−6 while the mole fraction of O3 in the air leaving the ozonizer is 0.02. Determine the molar (mol/min) and volumetric (m3/min) flow rate of the feed air and the rate of mass transfer of O3.
(c) Assume that the air bubbles in the ozonizer occupy a volume of 0.3 m3 and that they are, on overage, 2 mm in diameter.
Determine the mass transfer flux of O3 at the bubble–water interface.
FIGURE P.8.1:
Step by Step Answer:

Heat And Mass Transfer For Chemical Engineers Principles And Applications
ISBN: 9781264266678
1st Edition
Authors: Giorgio Carta





