Question:
Repeat Example 15-11 for the following conditions:
a. \(0.03 \mathrm{~g} \mathrm{CO}_{2} / 1000 \mathrm{~g}\) water in the drop. \(\mathrm{y}_{\text {water, bulk }}=0, \mathrm{y}_{\mathrm{CO} 2, \text { bulk }}=0.05\).
b. \(0.00 \mathrm{~g} \mathrm{CO}_{2} / 1000 \mathrm{~g}\) water in the drop. \(\mathrm{y}_{\text {water, bulk }}=0, \mathrm{y}_{\mathrm{CO} 2, \text { bulk }}=0.0007\).
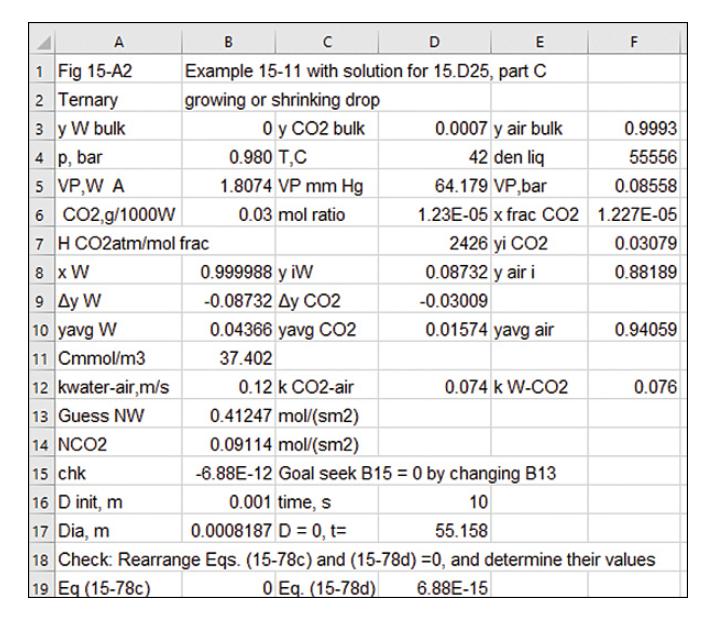
c. \(* 0.03 \mathrm{~g} \mathrm{CO}_{2} / 1000 \mathrm{~g}\) water in the drop. \(\mathrm{y}_{\text {water, bulk }}=0, \mathrm{y}_{\mathrm{CO} 2, \text { bulk }}=0.0007\).
* Answer is in a spreadsheet in Figure 15-A2.
Example 15-11
Figure 15-A2
Transcribed Image Text:
A drop of water containing CO2 is evaporating into air at 0.98 bar and 42C. The initial drop size is 1.0 mm. Report the mole fractions of air at the interface and in the bulk, the fluxes of all three components, the diameter D of the drop after 10s, and the time when the drop disappears for the following: a. 0.03 g CO2/1000 g water in the drop. Ywater, bulk = 0, yco2,bulk = 0.035. b. 0.03 g CO2/1000 g water in the drop. Ywater, bulk = 0.05, yco2,bulk = 0.035. Data: VP of water can be estimated from the Antoine equation (Table 2-3): logo (VP) 8.01767-1715.70/(T+234.268) where VP is in mm Hg and T in C. Henry's law coefficients for CO2 in water are listed in Table 12-1. Assume air is insoluble in water. Mass transfer coefficients are kw-air = 0.12 m/s, kco2-air = 0.074 m/s, and kco2-w = 0.076 m/s. TABLE 2-3. Constants for Antoine Eq. (2-9) Data from Various Sources (VP in mm Hg; T in C) Compound A B C Temperature Range, C Benzene 6.90565 1211.033 220.79 Benzene 6.87987 1196.76 219.161 8-80 Toluene 6.95087 1342.31 219.187 -27-111 n-Butane 6.809 935.86 238.73 n-Pentane 6.853 1064.8 233.01 n-Hexane 6.876 1171.17 224.41 Acetone 7.63132 1566.69 273.419 57-205 Methanol 8.08097 1582.271 239.726 15-84 Ethanol 8.11220 1592.864 226.184 20-93 Ethanol 8.32109 1718.10 237.52 -2-100 water 8.01767 1715.70 234.268 100-265 Methanol 5.20409 1581.341 -33.50 288.1-356.83 Ethanol 5.24677 1598.673 -46.424 292.77-366.63 1-Propanol 5.31384 1690.864 -51.804 292.4-370.5 Isopropanol 4.8610 1357.427 -75.814 329.92-362.41 1-Butanol 4.54607 1351.555 -93.34 295.8-391.0 2-Butanol 4.32943 1158.672 -104.683 354.54-380.30 1-Octanol 4.80915 1753.525 Water 4.6543 1435.264 -99.0 -64.848 328.02-387.0 255.9-373 TABLE 12-1. Henry's law constants H for Eq. (12-3) for CO2, CO, and H2S in water. H is in atm/mole fraction. TC 0 CO 728 CO HS 35,200 268 | 88 89 876 39,600 315 1040 44,200 367 1220 48,900 423 1420 53,600 483 1640 58,000 545 1860 62,000 609 2090 65,900 676 2330 69,600 745 2570 72,900 814 2830 76,100 884 3410 82,100 1030 100 | | | | 84,500 1190 84,500 1350 84,600 1440 84,600 1480









