The constants obtained by Shende and Sharma (1974) for use in Eq. (16-72) are given in the
Question:
The constants obtained by Shende and Sharma (1974) for use in Eq. (16-72) are given in the following table. Assume their experiments with \(\mathrm{NaOH}-\mathrm{SO}_{2}\) were done at \(1.0 \mathrm{~atm}\) and \(303 \mathrm{~K}\), and the gas is ideal.
a. Calculate \(\mathrm{k}_{\mathrm{p}}\) for each of their packings for \(\mathrm{u}_{\mathrm{G}}=200.0 \mathrm{~cm} / \mathrm{s}\) and \(\mathrm{u}_{\mathrm{L}}\) \(=3.0 \mathrm{~cm} / \mathrm{s}\).
b. Convert \(\mathrm{k}_{\mathrm{p}}\) to \(\mathrm{k}_{\mathrm{y}}\) and to \(\mathrm{k}_{\mathrm{c}}\).
c. For the operating conditions in part a, calculate the height of packing required for each of the three types of packing if a \(99.2 \%\) removal of \(\mathrm{SO}_{2}\) is desired.

Eq.(16-72)
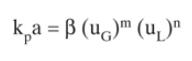
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





