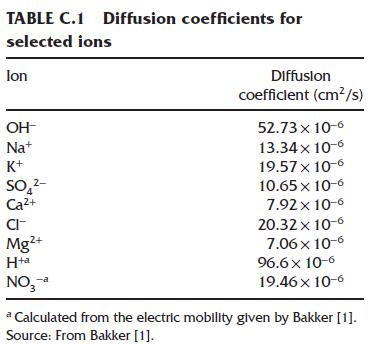
Question: Using the Henderson equation and table of diffusion coefficients in Appendix C, calculate the junction potential for a salt bridge with (3 mathrm{M} mathrm{KCl}) solution
Using the Henderson equation and table of diffusion coefficients in Appendix C, calculate the junction potential for a salt bridge with \(3 \mathrm{M} \mathrm{KCl}\) solution contacting a sample solution with \(0.1 \mathrm{M} \mathrm{NaOH}\).
Data from Henderson equation and table of diffusion coefficients in Appendix C
![E = = zjlzi]Dj (Cj.s-Cj,r) RT ID (GG) In (C.14) zlz |](https://dsd5zvtm8ll6.cloudfront.net/images/answer_images/1710/0/5/3/49165ed5873b8a691710053491458.jpg)
E = = zjlzi]Dj (Cj.s-Cj,r) RT ID (GG) In (C.14) zlz | DjCj.r
Step by Step Solution
3.52 Rating (159 Votes )
There are 3 Steps involved in it
From Eq C14 E where EDCC RT zz JD CjsCjr In 7 RT D C H zDCsC M z... View full answer

Get step-by-step solutions from verified subject matter experts


