We are separating a mixture of methanol and water in a flash drum at 1 atm pressure.
Question:
We are separating a mixture of methanol and water in a flash drum at 1 atm pressure. Equilibrium data are listed in Table 2-8.
a. Feed is 60.0 mol% methanol, and 40.0% of the feed is vaporized. What are the vapor and liquid mole fractions and flow rates? Feed rate is 100.0 kmol/h.
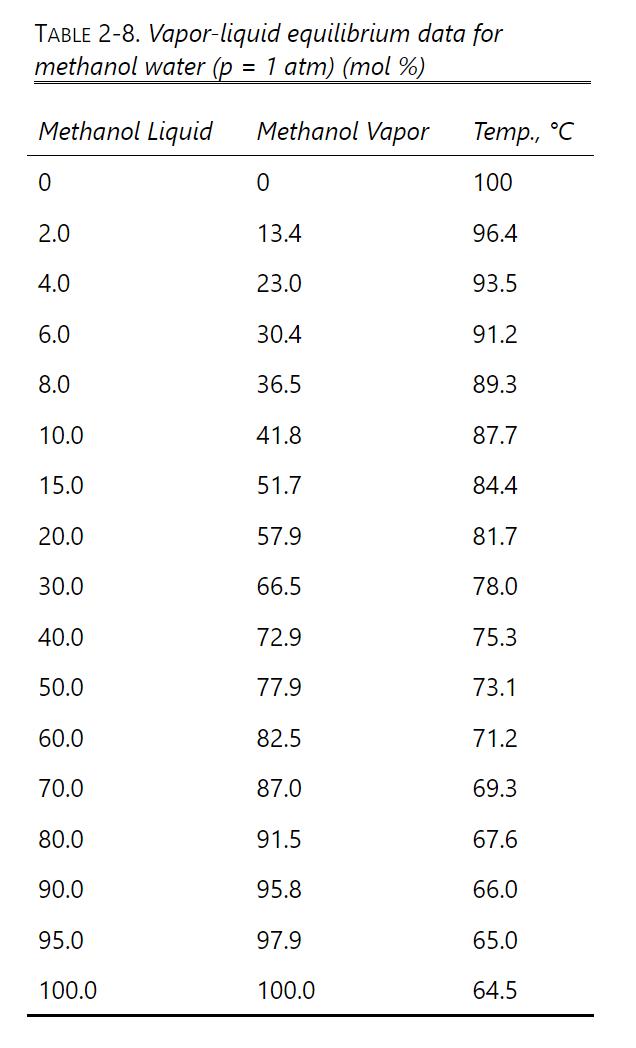
b. Repeat part a for a feed rate of 1500.0 kmol/h.
c. If the feed is 30.0 mol% methanol and we desire a liquid product that is 20.0 mol% methanol, what V/F must be used? For a feed rate of 1000.0 lbmol/h, find product flow rates and compositions.
d. We are operating the flash drum so that the liquid mole fraction is 45 mol% methanol. L = 1500.0 kmol/h, and V/F = 0.20. What must the flow rate and composition of the feed be?
e. Find the dimensions of a vertical flash drum for Problem 2.D1c. Data: Pw = 1.00 g/cm3, Pm,L = 0.7914 g/cm, MWW 18.01, MWm = 32.04. Assume vapors are ideal gas.
f. If z = 0.40, p = 1.0 atm, and Tdrum = 77.0C, find V/F, Xm, and Ym.
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





