We plan to treat (150.0 mathrm{kmol} / mathrm{h}) of water that is saturated with carbon tetrachloride (the
Question:
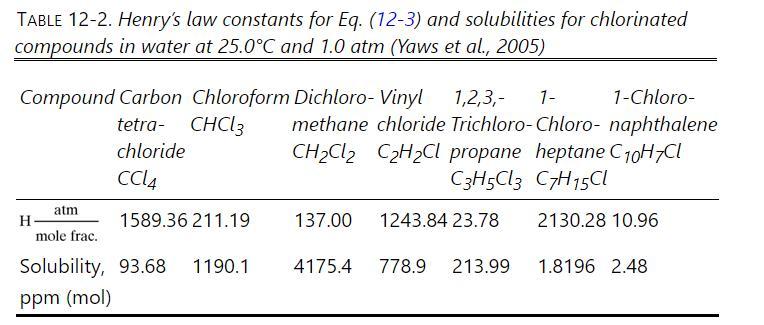
We plan to treat \(150.0 \mathrm{kmol} / \mathrm{h}\) of water that is saturated with carbon tetrachloride (the \(\mathrm{CCl}_{4}\) is at the solubility limit shown in Table \(12-2\) ) at \(25.0^{\circ} \mathrm{C}\) and a pressure of 0.96 bar by stripping with pure air at \(25.0^{\circ} \mathrm{C}\) and 0.96 bar (parts
a, b, c). Remove \(90.0 \%\) of the \(\mathrm{CCl}_{4}\). Assume the Henry's law constant and solubility of \(\mathrm{CCl}_{4}\) do not depend on pressure.
Table 12-2
a. What is the minimum value of gas flow rate \(\mathrm{V}_{\min }\) ?
b. If we operate at \(\mathrm{V}=2.5 \times \mathrm{V}_{\text {min }}\), what is the value of \(\mathrm{V}\), how many stages are needed, and what is the outlet gas mole fraction of \(\mathrm{CCl}_{4}\) ?
c. If we operate at \(\mathrm{V}=1.10 \times \mathrm{V}_{\text {min }}\), what is the value of \(\mathrm{V}\), how many stages are needed, and what is the outlet gas mole fraction of \(\mathrm{CCl}_{4}\) ?
d. Your boss wants to know what the minimum gas flow rate will be if the stripper is operated under a vacuum at 0.5 bar and \(25.0^{\circ} \mathrm{C}\).
e. If we operate at 0.5 bar and \(25.0^{\circ} \mathrm{C}\) and \(\mathrm{V}=2.5 \times \mathrm{V}_{\text {min }}\), what is the value of \(\mathrm{V}\), how many stages are needed, and what is the outlet gas mole fraction of \(\mathrm{CCl}_{4}\) ?
f. If we operate at \(0.5 \mathrm{bar}\) and \(25.0^{\circ} \mathrm{C}\) and \(\mathrm{V}=1.10 \times \mathrm{V}_{\min }\), what is the value of \(\mathrm{V}\), how many stages are needed, and what is the outlet gas mole fraction of \(\mathrm{CCl}_{4}\) ?
g. Explain how \(\mathrm{N}\) can be the same in parts \(\mathrm{b}\) and \(\mathrm{e}\) at different pressures, but \(\mathrm{y}_{\text {out }}\) is larger at the lower pressure.
h. Approximately, what is lowest pressure that can be used if temperature is \(25.0^{\circ} \mathrm{C} ?\)
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





