Question: Your task is to obtain the solution by analytical and numerical methods and compare it with the perturbation solution. Verify the following analytical solution for
Your task is to obtain the solution by analytical and numerical methods and compare it with the perturbation solution.
Verify the following analytical solution for the concentration distribution and the gradient at the gas-liquid interface:
\[\begin{equation*}c=c_{1}+\left(1-c_{1}+\frac{p_{1}}{K+1}\right) \frac{\sinh [\beta(1-x)]}{\sinh \beta}+\frac{p_{1}}{K+1}(x-1) \tag{14.56}\end{equation*}\]
and
\[\begin{equation*}p_{1}=-\frac{\left(1-c_{1}\right)(1+K)}{1+(K / \beta) \tanh \beta} \tag{14.57}\end{equation*}\]
These equations can be used to compare the numerical results as well as perturbation results. Perform such a comparison for values of \(M\) and \(K\) in the text and verify the results in Table 14.2.
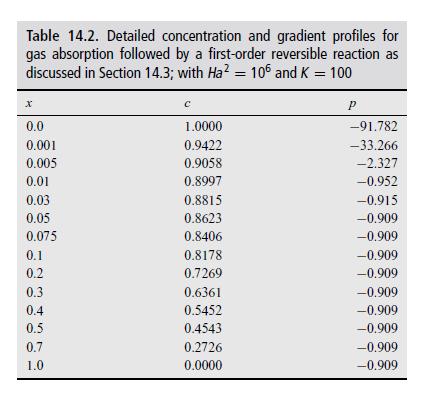
Table 14.2. Detailed concentration and gradient profiles for gas absorption followed by a first-order reversible reaction as discussed in Section 14.3; with Ha = 106 and K = 100 x 0.0 P 1.0000 -91.782 0.001 0.9422 -33.266 0.005 0.9058 -2.327 0.01 0.8997 -0.952 0.03 0.8815 -0.915 0.05 0.8623 -0.909 0.075 0.8406 -0.909 0.1 0.8178 -0.909 0.2 0.7269 -0.909 0.3 0.6361 -0.909 0.4 0.5452 -0.909 0.5 0.4543 -0.909 0.7 0.2726 -0.909 1.0 0.0000 -0.909
Step by Step Solution
3.28 Rating (154 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


