Your technician is doing a steady-state evaporative crystallization of nickel chloride. The inlet water flow rate is
Question:
Your technician is doing a steady-state evaporative crystallization of nickel chloride. The inlet water flow rate is \(100 \mathrm{~kg} / \mathrm{h}\). The feed is a saturated liquid at \(100^{\circ} \mathrm{C}\) and the evaporative crystallizer also operates at \(100^{\circ} \mathrm{C}\). The vapor and entrained liquid removed is condensed and weighed, and we find there is \(20.5 \mathrm{~kg} / \mathrm{h}\) of liquid. The technician noticed that the condensed liquid is salty. Upon measurement, he found that the concentration of anhydrous \(\mathrm{NiCl}_{2}\) in the vapor plus entrained liquid that was then condensed is \(2.20 \mathrm{~kg} / 100 \mathrm{~kg}\) water. Solubility data are in Table 17-1.
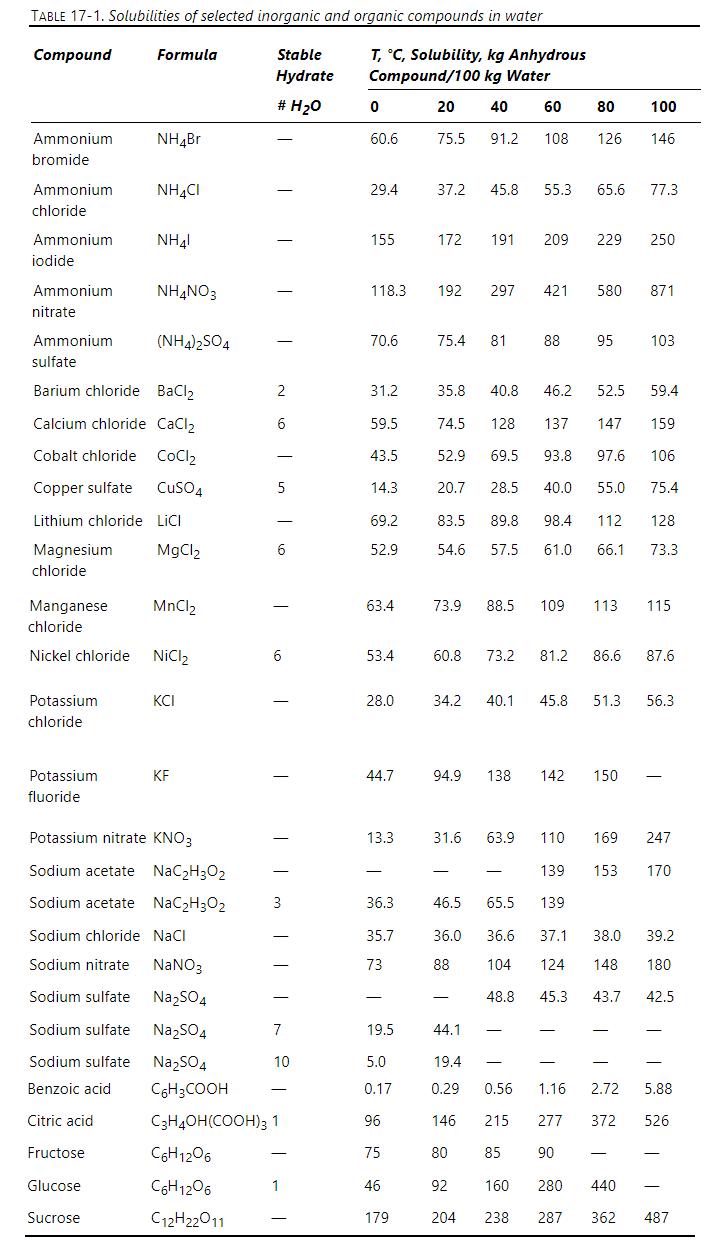
a. How many \(\mathrm{kg} / \mathrm{h}\) of anhydrous \(\mathrm{NiCl}_{2}, \mathrm{~A}\), precipitate in the crystallizer?
b. How many \(\mathrm{kg} / \mathrm{h}\) of the stable hydrate, \(\mathrm{NiCl}_{2} \cdot 6 \mathrm{H}_{2} \mathrm{O}\), precipitate in the crystallizer?
c. What is \(\mathrm{F}_{\mathrm{W}, \text { out }}\) in \(\mathrm{kg} / \mathrm{h}\) ?
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





