A vaporizer is required to evaporate 10,000 kg/h of a process fluid, at 6 bar. The liquid
Question:
A vaporizer is required to evaporate 10,000 kg/h of a process fluid, at 6 bar. The liquid is fed to the vaporizer at 20°C.
The plant has a spare kettle reboiler available with the following specification. U-tube bundle, 50 tubes, mean length 4.8 m, end to end. Carbon steel tubes, inside diameter 25 mm, outside diameter 30 mm, square pitch 45 mm.
Transcribed Image Text:
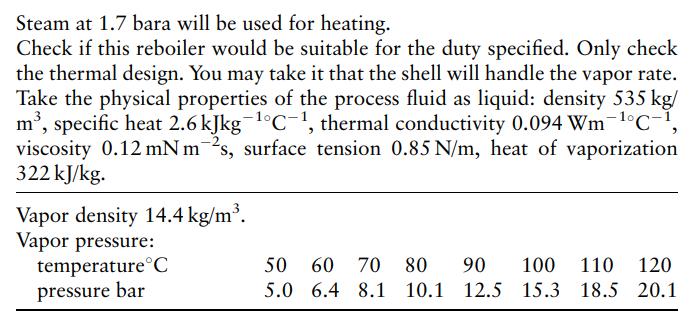
Steam at 1.7 bara will be used for heating. Check if this reboiler would be suitable for the duty specified. Only check the thermal design. You may take it that the shell will handle the vapor rate. Take the physical properties of the process fluid as liquid: density 535 kg/ m³, specific heat 2.6 kJkg-¹°C-1, thermal conductivity 0.094 Wm-¹°C-1, viscosity 0.12 mNm 2s, surface tension 0.85 N/m, heat of vaporization 322 kJ/kg. Vapor density 14.4 kg/m³. Vapor pressure: temperature C pressure bar 50 60 70 80 90 100 110 120 5.0 6.4 8.1 10.1 12.5 15.3 18.5 20.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
Thermal Design Check of Kettle Reboiler Given Process fluid to be evaporated 10000 kgh Pressure 6 ba...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
A pure fluid is described by the Antoine equation With P in bar and T in kelvin. The density of the liquid at room temperature is 0.656 g/cm 3 . How much heat is required to evaporate 1 kg of that...
-
HR Trends Institute https://hrtrendinstitute.com/(Links to an external site.) HR Technology Discuss IT changes that you have experienced at work such as new cash register or inventory system or...
-
Evan Root operates a bowling alley. He has just received the monthly bank statement at April 30 from City National Bank, and the statement shows an ending balance of $565. Listed on the statement are...
-
In December, Koala Camp Gear Company produced 2,000 Tree Line tents and incurred the following actual Decision costs for direct material and direct labor: MEL g Purchased 25,000 sq. meters of tent...
-
Combine the classification results into voting ensemble models, using the following methods: a. Majority classification b. Single sufficient classification c. Twofold sufficient classification d....
-
Sandy Sally is a sole proprietor CPA who runs a successful practice with five employees. Several years ago, Sally purchased an office building and relocated the practice in about 20 percent of the...
-
In activity - based costing, what is the primary purpose of using multiple cost drivers? What are the total direct labor hours for: Computer desk = L - shaped desk = U - shaped desk = Total direct...
-
A horizontal, cylindrical tank, with hemispherical ends, is used to store liquid chlorine at 10 bar. The vessel is 4 m internal diameter and 20 m long. Estimate the minimum wall thickness required to...
-
Discuss whether the passive activity loss rules apply to the following: individuals, closely held C corporations, S corporations, partnerships, and personal service corporations.
-
Based on the information below for Maize Industries LLC, construct a statement of financial position, statement of income, statement of changes in equity and statement of cash flows for the year...
-
Suppose that your credit card activity for December looked like this: Date Activity December 5 $384 purchase December 11 $347 purchase December 16 $174 purchase December 21 $480 purchase December 25...
-
A research group surveyed 300 students. The students were asked how often they go to the movies and whether they prefer comedies or dramas. Their responses are summarized in the following table....
-
C. Prove the following (you can use any formal induction/other theoretical method, "A" means power here): i. ii. iii. What is the time complexity recurrence relation for Fibonacci numbers? Explain it...
-
You and your partner run a small business together, with separate work roles. You are responsible for the business budget and have researched an improved budget process which you felt needs to be...
-
The actual selling expenses incurred in March 2022 by Carla Vista Company are as follows: Variable Expenses Fixed Expenses Sales commissions Advertising $14,576 Sales salaries $34,700 12.174...
-
Include appropriate units in all answers. a. Find the mean for each of the three flights. b. Find the standard deviation for each of the three flights. c. Find the variance for each of the three...
-
2.) Find the Laplace transform of f(t) 7e-St cos 2t +9 sinh2 2t. Use Laplace Table. %3D
-
In the manufacture of vinyl chloride (VC) by the pyrolysis of dichloroethane (DCE), the reactor conversion is limited to 55% to reduce carbon formation, which fouls the reactor tubes. Calculate the...
-
In the production of ethanol by the hydrolysis of ethylene, diethyl ether is produced as a by-product. A typical feed stream composition is: 55% ethylene, 5% inerts, 40% water; and product stream:...
-
In the chlorination of ethylene to produce dichloroethane (DCE), the conversion of ethylene is reported as 99.0%. If 94 mol of DCE are produced per 100 mol of ethylene reacted, calculate the...
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...

Study smarter with the SolutionInn App


