Aniline is produced by the hydrogenation of nitrobenzene. A small amount of cyclo-hexylamine is produced as a
Question:
Aniline is produced by the hydrogenation of nitrobenzene. A small amount of cyclo-hexylamine is produced as a byproduct. The reactions are
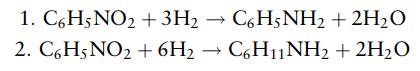
Nitrobenzene is fed to the reactor as a vapor, with three times the stoichiometric quantity of hydrogen. The conversion of the nitrobenzene, to all products, is 96%, and the selectivity for aniline is 95%.
The unreacted hydrogen is separated from the reactor products and recycled to the reactor. A purge is taken from the recycle stream to maintain the inerts in the recycle stream below 5%. The fresh hydrogen feed is 99.5% pure, the remainder being inerts. All percentages are molar.
For a feed rate of 100 kmol/h of nitrobenzene, calculate
i. The fresh hydrogen feed;
ii. The purge rate required;
iii. The composition of the reactor outlet stream.
Step by Step Answer:






