Calculate the standard heat of the following reaction, given the enthalpies of formation: Standard enthalpies of formation
Question:
Calculate the standard heat of the following reaction, given the enthalpies of formation:
![]()
Standard enthalpies of formation kJ/mol
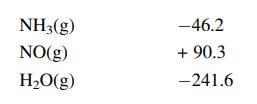
Transcribed Image Text:
4NH3(g) +50₂(g) →4NO(g) + 6H₂O(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
The enthalpy of formation ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Engineering Design
ISBN: 9780081025994
6th Edition
Authors: Ray Sinnott, R.K. Sinnott, Sinnott Gavin Towler
Question Posted:
Students also viewed these Engineering questions
-
You have been given the task of determining the standard heat of the reaction in which calcium chloride hexahydrate is formed from anhydrous calcium chloride: CaCl 2 (s) + 6 H 2 O (l) CaCl 2 6H 2...
-
Calcium chloride is a salt used in a number of food and medicinal applications and in brine for refrigeration systems. Its most distinctive property is its affinity for water: in its anhydrous form...
-
Trichloroethylene, a widely used degreasing solvent for machine parts, is produced in a two-step reaction sequence. Ethylene is first chlorinated to yield tetrachloroethane, which is...
-
1-Is it a good business strategy to have a day care center, Harley Davidson Motorcycle Dealership Sales and repair, Namaste Yoga studio, bookstore, temporary worker (day laborer) center, state prison...
-
The following paragraphs appeared in the New York Times on September 22, 1986. To keep the dollar from falling against the West German mark, the European central banks would have to sell marks and...
-
The crate has a weight W and a center of gravity at G. Determine the height h of the tow rope so that the crate slips and tips at the same time. What horizontal force P is required to do this? Given:...
-
REPLACEMENT ANALYSIS The Chang Company is considering the purchase of a new machine to replace an obsolete one. The machine being used for the operation has a book value and a market value of zero....
-
The actual selling expenses incurred in March 2017 by Fallon Company are as follows. Instructions (a) Prepare a flexible budget performance report for March using the budget data in E24-5, assuming...
-
Selected balance sheet information for the Wolf Company at November 30, and December 31, 2021, is presented below. The company uses the perpetual inventory system and all sales to customers are made...
-
Hydrogen chloride gas, produced by burning chlorine with hydrogen, is required at a supply pressure of 600 kN/m 2 , gauge. The pressure can be achieved by either operating the burner under pressure...
-
Hydrogen chloride is produced by burning chlorine with an excess of hydrogen. The reaction is highly exothermic and reaches equilibrium very rapidly. The equilibrium mixture contains approximately 4...
-
The report Trends in Higher Education (www. college board.com) gave the accompanying data on smoking rates for people age 25 and older by education level. a. Construct a time series plot for people...
-
Making Consumer Choices The Espresso Machine (25 points) In real life, you must often make choices about whether to buy something pre-made or make it yourself. There are many things to consider:...
-
1) Read over the article/case and summarize what it is referring to in your own words. 2) What type of leadership traits can you describe in the case study? Use materials both from the handout and...
-
After reading or watching, https://smallbusiness.chron.com/internal-analysis-important-80513.html https://www.indeed.com/career-advice/career-development/internal-analysis...
-
At the Business Level there are a couple main strategies that companies use- Cost Leadership and Differentiation. What is the difference between them? Share some examples of companies or specific...
-
https://youtu.be/c_Eutci7ack After watching the video, what are your thoughts on Power? Would you want to have this Power ? Why would you not want this Power? If you are a manager or want to be a...
-
Indicate in which financial statement each of the following adjusted trial balance yet account would be presented. Service Revenue ....................... Accounts Receivable Notes Payable...
-
A Firm intends to invest some capital for a period of 15 years; the Firm's Management considers three Options, each consisting of purchasing a machinery of a specific brand, different for each...
-
Suggest suitable materials of construction for the following applications: 1. A 10,000 m 3 storage tank for toluene; 2. A 5:0 m 3 tank for storing a 30% w/w aqueous solution of sodium chloride; 3. A...
-
Choose a suitable material of construction for the following duties: 1. 98% w/w sulfuric acid at 708C; 2. 5% w/w sulfuric acid at 308C; 3. 30% w/w hydrochloric acid at 508C; 4. 5% aqueous sodium...
-
A pipeline constructed of carbon steel failed after 3 years of operation. On examination it was found that the wall thickness had been reduced by corrosion to about half the original value. The...
-
Please give an accurate and complete answer. Please pay attention to the requirements in red. If correct, I will give a good comment. Required Information [The following information applies to the...
-
On August 31, Year 1, the general ledger of a company shows a balance for cash of $7,864. Cash receipts yet to be deposited into the checking account total $3,258, and checks written by the academy...
-
Hance Lane, is preparing for a meeting with investors. He would like to provide appropriate information about his companys financial position. Lance has provided the following accounts and balances...

Study smarter with the SolutionInn App


