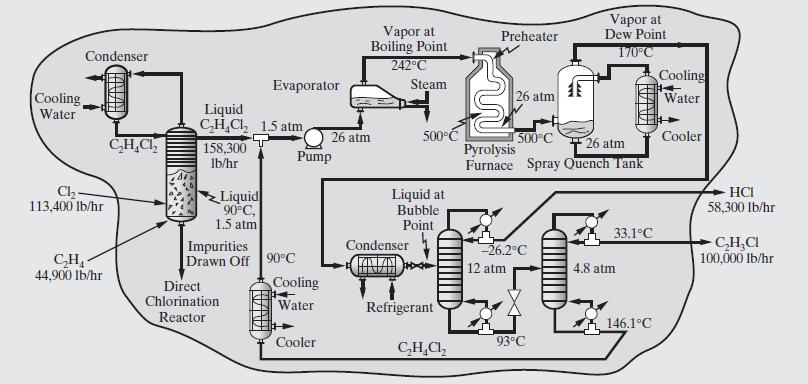
Consider the flowsheet for the manufacture of vinyl chloride in Figure 2.6. (a) If the pyrolysis furnace
Question:
Consider the flowsheet for the manufacture of vinyl chloride in Figure 2.6.
(a) If the pyrolysis furnace and distillation towers are operated at low pressure \((1.5 \mathrm{~atm})\), what are the principal disadvantages? What alternative means of separation could be used?
(b) For the process shown, is it possible to use some of the heat of condensation from the \(\mathrm{C}_{2} \mathrm{H}_{4} \mathrm{Cl}_{2}\) to drive the reboiler of the first distillation tower? Explain your response. If not, what process change would make this possible?
(c) Consider the first reaction step to make dichloroethane. Show the distribution of chemicals when ethylene is \(20 \%\) in excess of the stoichiometric amount and the chlorine is entirely converted. Assume that \(100,000 \mathrm{lb} / \mathrm{hr}\) of vinyl chloride are produced.
(d) Consider the first distillation tower. What is the advantage of cooling the feed to its bubble point at \(12 \mathrm{~atm}\) as compared with introducing the feed at its dew point?
(e) Why isn't the feed to the pyrolysis furnace heated with the hot pyrolysis products?
(f) What is the function of the trays in the direct chlorination 3 reactor?
(g) Suggest ways to reduce the need for fuel and hot utilities such as steam.
Figure 2.6:-
Step by Step Answer:

Product And Process Design Principles Synthesis Analysis And Evaluation
ISBN: 9781119355243
4th Edition
Authors: Warren D. Seider, Daniel R. Lewin, J. D. Seader, Soemantri Widagdo, Rafiqul Gani, Ka Ming Ng





