For the reaction system: [begin{aligned}& mathrm{A}+mathrm{B} xrightarrow{k_{1}} mathrm{C}+mathrm{A} xrightarrow{k_{3}} mathrm{E} & bigvee_{2} mathrm{D}+mathrm{A} earrowend{aligned}] select an operating
Question:
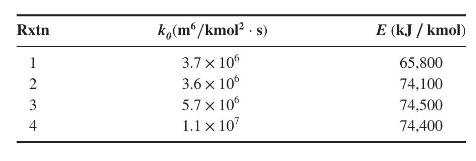
For the reaction system:
\[\begin{aligned}& \mathrm{A}+\mathrm{B} \xrightarrow{k_{1}} \mathrm{C}+\mathrm{A} \xrightarrow{k_{3}} \mathrm{E} \\& \bigvee_{2} \mathrm{D}+\mathrm{A} earrow\end{aligned}\]
select an operating temperature that favors the production of \(\mathrm{C}\). The pre-exponential factor and activation energy for the reactions are tabulated as follows:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Product And Process Design Principles Synthesis Analysis And Evaluation
ISBN: 9781119355243
4th Edition
Authors: Warren D. Seider, Daniel R. Lewin, J. D. Seader, Soemantri Widagdo, Rafiqul Gani, Ka Ming Ng
Question Posted:





