In Example 13.1, an absorber with an absorbent rate of (237 mathrm{kmol} / mathrm{hr}) and 4 equilibrium
Question:
In Example 13.1, an absorber with an absorbent rate of \(237 \mathrm{kmol} / \mathrm{hr}\) and 4 equilibrium stages absorbs \(90 \%\) of the entering \(n\)-butane. Repeat the calculations for:
(a) \(474 \mathrm{kmol} / \mathrm{hr}\) of absorbent (twice the flow) and four equilibrium stages.
(b) Eight equilibrium stages (twice the stages) and \(237 \mathrm{kmol} / \mathrm{hr}\) of absorbent.
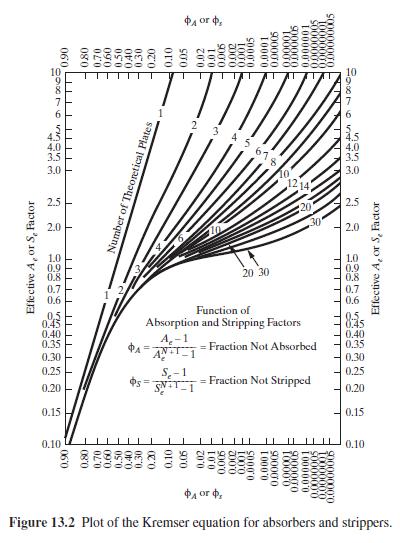
Which case results in the most absorption of \(n\)-butane? Is this result confirmed by the trends of the curves in the Kremser plot of Figure 13.2.
Data From Example 13.1:-

Figure 13.2:-
Step by Step Answer:
Related Book For 

Product And Process Design Principles Synthesis Analysis And Evaluation
ISBN: 9781119355243
4th Edition
Authors: Warren D. Seider, Daniel R. Lewin, J. D. Seader, Soemantri Widagdo, Rafiqul Gani, Ka Ming Ng
Question Posted:




