Calculate the concentrations of Ag + , Ag(S 2 O 3 ) , and Ag(S 2
Question:
Calculate the concentrations of Ag+, Ag(S2O3)–, and Ag(S2O3)23– in a solution prepared by mixing 150.0 mL of 1.00 × 10–3 M AgNO3 with 200.0 mL of 5.00 M Na2S2O3. The stepwise formation equilibria are
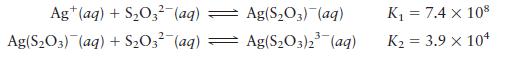
Transcribed Image Text:
Ag+ (aq) + SO3 (aq) Ag(S03) (aq) + SO3- (aq) Ag(S03) (aq) Ag(SO3)2(aq) K = 7.4 x 108 K = 3.9 x 10
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
The concentrations of the ligand and metal ion in the mixed solu tion before any reaction occurs are ...View the full answer

Answered By

Saud Ur Rehman
Evaluating manufacturing processes by designing and conducting research programs; applying knowledge of product design, fabrication, assembly, tooling, and materials; conferring with equipment vendors; soliciting observations from operators. Developing manufacturing processes by studying product requirements; researching, designing, modifying, and testing manufacturing methods and equipment; conferring with equipment vendors. Keeping equipment operational by coordinating maintenance and repair services; following manufacturer's instructions and established procedures; requesting special service.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the equilibrium concentrations of NH 3 , Cu 2+ , Cu(NH 3 ) 2+ , Cu(NH 3 ) 2 2+ , Cu(NH 3 ) 3 2+ , and Cu(NH 3 ) 4 2+ in a solution prepared by mixing 500.0 mL of 3.00 M NH 3 with 500.0 mL...
-
The solubility-product constant for Ce(IO3)3 is 3.2 x 10-10. What is the Ce3+ concentration in a solution prepared by mixing 50.00 mL of 0.0450 M Ce3+ with 50.00 mL of? (a) Water? (b) 0.0450 M IO3-?...
-
Calculate [HY3-] in a solution prepared by mixing 10.00 mL of 0.010 0 M VOSO4, 9.90 mL of 0.010 0 M EDTA, and 10.0 mL of buffer with a pH of 4.00.
-
According to Electronic Designs 2012 Engineering Salary Survey, the mean base salary of a software engineering manager is $126,417the highest mean among all types of engineers. In contrast, a...
-
A photon is scattered at an angle 0 = 120 by a stationary free electron. As a result, the electron acquires a kinetic energy T = 0.45 MeV. Find the energy that the photon had prior to scattering.
-
When a futures contract is traded on the floor of the exchange, it may be the case that the open interest increases by one, stays the same, or decreases by one. Explain this statement.
-
Define the business vision and objectives.
-
Calvin Hughess will provide for the following distributions: a. The 40-acre parcel in Leona, Wisconsin, is to be given to The Nature Conservancy along with $50,000. b. My 1970 GTO Pontiac convertible...
-
the part of the sales budget for the next three month period, the Alderman Company determined that 44.000 liked it would be needed to selections. The company has any of 11.000 he good on hand at...
-
You have two salts \(\mathrm{AgX}\) and \(\mathrm{AgY}\) with very similar \(K_{\mathrm{sp}}\) values. You know that the \(K_{\mathrm{a}}\) value for \(\mathrm{HX}\) is much greater than the...
-
A solution contains 1.0 10 4 M Cu + and 2.0 10 3 M Pb 2+ . If a source of I is added to this solution gradually, will PbI 2 (K sp = 1.4 10 8 ) or CuI (K sp = 5.3 10 12 ) precipitate first?...
-
Solve the following problem and express the answer in meters with the appropriate number of significant figures and in scientific notation: 3.08 101 km + 2.00 103 cm
-
A popular theory is that presidential candidates have an advantage if they are taller than their main opponents. Listed are heights (in centimeters) of randomly selected presidents along with the...
-
Cash Flows Horiz Analysis Horiz Analysis Vertic Analysis Vertic Analysis from Oper Inc St Bal St Inc St Bal Sheet Ratios Requirement Prepare the cash flows from operations section of R. Ashburn...
-
Rudy Gandolfi owns and operates Rudy's Furniture Emporium Incorporated. The balance sheet totals for assets, liabilities, and stockholders' equity at August 1, 2022, are as indicated. Described here...
-
The brand manager for a brand of toothpaste must plan a campaign designed to increase brand recognition. He wants to first determine the percentage of adults who have heard of the brand. How many...
-
Pulse rates of women are normally distributed with a mean of 77.5 beats per minute and a standard deviation of 11.6 beats per minute. Answer the following questions. What are the values of the mean...
-
How does a work group have control over the performance of a worker? Provide a rationale for why this "power" is a positive or negative thing?
-
Time Solutions, Inc. is an employment services firm that places both temporary and permanent workers with a variety of clients. Temporary placements account for 70% of Time Solutions' revenue;...
-
Calculate the density of each of the following metals from the data given: (a) Aluminum, fcc structure, atomic radius 143 pm; (b) Potassium, bcc structure, atomic radius 227 pm.
-
Calculate the pressure exerted by 1.00 mol H 2 S, behaving as (a) An ideal gas; (b) A van der Waals gas when it is confined under the following conditions: (i) At 273.15 K in 22.414 L; (ii) At 800. K...
-
Suppose that 0.473 g of an unknown gas that occupies 200. mL at 1.81 atm and 25C was analyzed and found to contain 0.414 g of nitrogen and 0.0591 g of hydrogen. (a) What is the molecular formula of...
-
Palisade Creek Co. is a merchandising business that uses the perpetual inventory system. The account balances for Palisade Creek Co. as of May 1, 2019 (unless otherwise indicated), are as follows:...
-
1-When accounting for an acquisition, goodwill is the difference between what two things? 2- What factors should be considered when deciding whether an acquisition should be financed with cash or...
-
What is the main friction Fluidity aims to address? REAL STATE

Study smarter with the SolutionInn App


