Sketch a galvanic cell, and explain how it works. Look at Figs. 11.1 and 11.2. Explain what
Question:
Sketch a galvanic cell, and explain how it works. Look at Figs. 11.1 and 11.2. Explain what is occurring in each container and why the cell in Fig. 11.2 “works” but the one in Fig. 11.1 does not.
Fig. 11.1
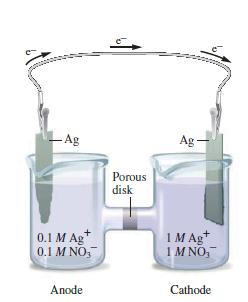
Fig. 11.2
Transcribed Image Text:
-Ag + 0.1 M Ag 0.1 M NO, Anode Porous disk Ag - 1 M Ag+ IMNO, Cathode
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 38% (13 reviews)
A galvanic cell also known as a voltaic cell is a type of electrochemical cell that converts chemica...View the full answer

Answered By

NARESH KUMAR
So the question became, how do I keep tutoring and help more people, a.k.a., clone myself. I got my answer teaching a team in Bangalore, India about pension plans. I built Excel spreadsheets with formulas. But, I added one twist…next to the number cells, I added comments with step-by-step math to explain actuarial formulas and life expectancies.
The team in Bangalore loved it, it cut their learning curve down significantly. So the lightbulb went on…why can’t I build a program/website to tutor in math. Essentially, can’t I put my brain on a website. This way, people can use the calculators without me having to be there.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What is a Kanban system? Explain how it works in a manufacturing setting. Also, describe how the Kanban system can be used to lower inventory levels.
-
Explain what a p-value is, and explain how it is used to test a hypothesis.
-
Explain what a critical value is, and explain how it is used to test a hypothesis.
-
Consider an increasing marginal-cost depletable resource with no effective substitute. (a) Describe, in general terms, how the marginal user cost for this resource in the earlier time periods would...
-
Find the z-score that has 78.5% of the distributions area to its left.
-
Answer each of the following questions. (a) What is the percentage rate if the base is 88 and the percentage is 55? (b) 63 is what percent of 36? (c) What is 3/4% of $64? (d) 450% of $5 is what...
-
On an exam with a mean of M 5 39, you obtain a score of X 5 35. a. Would you prefer a standard deviation of s 5 2 or s 5 8? (Hint: Sketch each distribution and find the location of your score.) b. If...
-
Mid-States Metal Finishers produces steel tubing at its Akron plant. The plants quality assurance officer prepares a monthly report and sends it to headquarters. The data compiled in these reports...
-
A manufacturing company applies factory overhead based on direct labor hours. At the beginning of the year, it estimated that factory overhead costs would be $412,695 and direct labor hours would be...
-
A nursing home employs attendants who are needed around the clock. Each attendant is paid the same, regardless of when his or her shift begins. Each shift is 8 consecutive hours. Shifts begin at 6...
-
Which of the following is the best reducing agent: F 2 , H + , Na, Na + , or F 2 ? Explain. Order as many of these species as possible from the best to the worst oxidizing agent. Why cant you order...
-
For each of the following pairs of elements, choose the one that correctly completes the following table. The more favor- able (exothermic) electron affinity The higher ioniza- tion energy The larger...
-
Determine whether the series is convergent or divergent. n=1 1.3.5. . (2n-1) 5*n!
-
What are the different types of drones?
-
What are the applications of drones?
-
What are the various protocols in telecom domain?
-
What are the various types of routing protocols?
-
For all the benefits they bring to business, social media and other communication technologies have created a major new challenge: responding to online rumors and attacks on a company's reputation....
-
These summary exercises provide practice with some of the concepts covered so far in this chapter. For the points P and Q, find (a) The distance d(P, Q), (b) The coordinates of the midpoint of the...
-
The rate at which the temperature of an object changes is proportional to the difference between its own temperature and the temperature of the surrounding medium. Express this rate as a function of...
-
Consider the couple Ox + e Red with the oxidized and redu ced species at unit activity. What must be the value of E for this half-cell if the reductant Red is to liberate hydrogen at 1 atm from a....
-
By finding appropriate half-cell reactions, calculate the equilibrium constant at 298.15 K for the following reactions: a. 4NiOOH(s) + 2 2 O(l) 4Ni(OH) 2 (s) + O 2 (g) b. 4NO 3 (aq)+ 4H + (aq)...
-
The cell potential E for the cell Pt(s)|H 2 (g, a H2 = 1) H + (aq, a H+ = 1)NaCl(aq, m = 0.300) AgCl(s) Ag(s) is +0.260 V. Determine Cl assuming that = Na+ = Cl .
-
DETAILS 1. [-/1 Points) SMITHNM13 11.2.025. MY NOTES Convert the credit card rate to the APR. Oregon, 2% per month % Need Help? ReadIt Watch
-
Corom Stack Standard CALCULATOR PRINTER VERSION BACK NEXT Problem 13-02A a-c (Part Level Submission) Sheffield Corporation had the following stockholders' equity accounts on January 1, 2020: Common...
-
Suppose that you own 2,100 shares of Nocash Corp. and the company is about to pay a 25% stock dividend. The stock currently sells at $115 per share. a. What will be the number of shares that you hold...

Study smarter with the SolutionInn App


