The four flasks below were prepared with the same volume and temperature. Flask I contains He atoms,
Question:
The four flasks below were prepared with the same volume and temperature. Flask I contains He atoms, Flask II contains Cl2 molecules, Flask III contains Ar atoms, and Flask IV contains NH3 molecules. Which flask has
(a) The largest number of atoms;
(b) The highest pressure;
(c) The greatest (mass) density;
(d) Molecules or atoms with the highest root mean square speed;
(e) Molecules or atoms with the highest molar kinetic energy?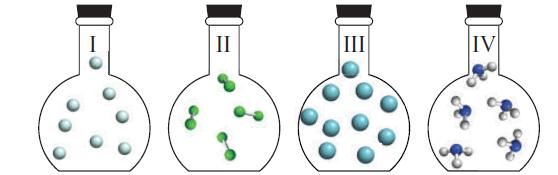
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





