Use data in Table 4C.1 or Appendix 2A to calculate the entropy change for (a) The freezing
Question:
Use data in Table 4C.1 or Appendix 2A to calculate the entropy change for
(a) The freezing of 1.00 mol H2O(l) at 0.00°C;
(b) The vaporization of 50.0 g of ethanol, C2H5OH, at 351.5 K.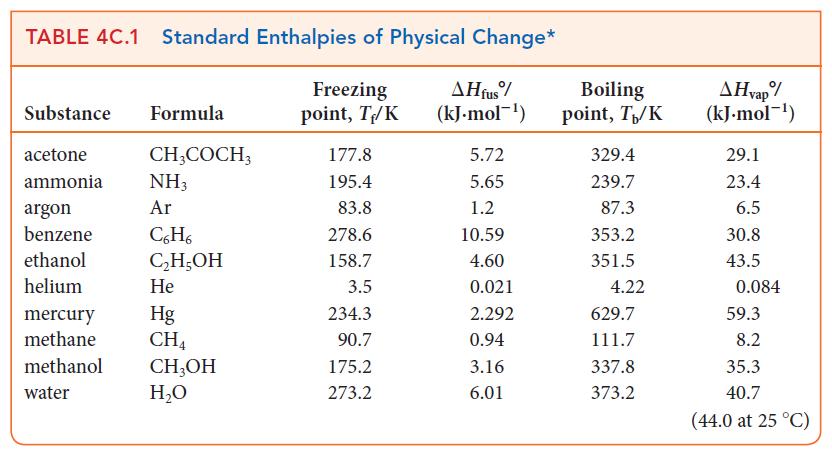
Transcribed Image Text:
TABLE 4C.1 Standard Enthalpies of Physical Change* Freezing AH fus% (kJ.mol-¹) point, T/K Substance Formula acetone ammonia argon benzene ethanol helium CH3COCH3 NH3 Ar C6H6 C₂H5OH He mercury Hg methane CH4 methanol CH3OH water H₂O 177.8 195.4 83.8 278.6 158.7 3.5 234.3 90.7 175.2 273.2 5.72 5.65 1.2 10.59 4.60 0.021 2.292 0.94 3.16 6.01 Boiling point, T₁/K 329.4 239.7 87.3 353.2 351.5 4.22 629.7 111.7 337.8 373.2 AHvap% (kJ.mol-¹) 29.1 23.4 6.5 30.8 43.5 0.084 59.3 8.2 35.3 40.7 (44.0 at 25 °C)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a 2...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use data in Table 4H.1 or Appendix 2A to calculate the standard reaction entropy for each of the following reactions at 25C. For each reaction, interpret the sign and magnitude of the reaction...
-
Use data in Table 4H.1 or Appendix 2A to calculate the standard entropy change for each of the following reactions at 25C. For each reaction, interpret the sign and magnitude of the reaction entropy....
-
Calculate the entropy change for the vaporization of liquid methane and hexane using the following data: Compare the molar volume of gaseous methane at 112 K with that of gaseous hexane at 342 K. How...
-
How do items discussed in the critical audit matters section differ from items in an unqualified opinion with an emphasis-of-matter paragraph? Question 1: (20) A. On November 1, 2019. James Andersun...
-
(a) What source documents are used in assigning (1) materials and (2) labor to production in a process cost system? (b) What criterion and basis are commonly used in allocating overhead to processes?
-
Evaluate the integral. sin e + tan 0 - de cos'e
-
P 16-4 Partnership income allocationComplex, net loss The partnership agreement of Ale, Car, and Eri provides that profits are to be divided as follows: 1. Ale is to receive a salary allowance of...
-
Your firm is contemplating the purchase of a new $410,000 computer-based order entry system. The system will be depreciated straight-line to zero over its five-year life. It will be worth $30,000 at...
-
Derrick Iverson is a divisional manager for Holston Company. His annual pay raises are largely determined by his divisions return on investment (ROI), which has been above 25% each of the last three...
-
Calculate the work for each of the following processes beginning with a gas sample in a piston assembly with T = 305 K, P = 1.79 atm, and V = 4.29 L: (a) Irreversible expansion against a constant...
-
What might you expect the residual molar entropy of trigonal pyramidal PH 2 F to be if it were to adopt an orientationally disordered arrangement in its crystal form?
-
The moment of inertia of a uniform rod about an axis through its center is 1/12 mL 2 . The moment of inertia about an axis at one end is 1/3 mL 2 . Explain why the moment of inertia is larger about...
-
Operating data for Oriole Corporation are presented as follows. 2025 2024 Net sales $831,900 $629,500 Cost of goods sold 529,700 410,900 Selling expenses 125,400 74,300 Administrative expenses 79,400...
-
A bird traveled 72 miles in 6 hours flying at constant speed. At this rate, how many miles did the bird travel in 5 hours? 12 O 30- O 60 14.4 I don't know (I need help with these type of questions)
-
A common trade off related to financial services include a. minimum deposit vs. maximum deposit b. availability vs. liquidity c. liquidity vs. access to funds d. convenience vs. fees e. RRSP vs RESP
-
Question 2. You won a free ticket to a Bruce Springsteen concert (next Friday) in a lottery. However, Rihanna is singing on the same evening. A ticket to the Rihanna concert is priced at $100 though...
-
Assignment 3 This assignment is based on content discussed in modules 3 - 5 and test basic concepts of statistical inference theory and probability distributions. Learning outcomes Work on problems...
-
Among different species, describe three distinct strategies for accomplishing dosage compensation.
-
What are some of the possible sources of information about a company that could be used for determining the companys competitive stance?
-
In 1994 chemists at Texas A&M University reported the synthesis of a non-naturally occurring amino acid: a. To which naturally occurring amino acid is this compound most similar? b. A tetrapeptide,...
-
Consider the following reactions at some temperature: 2NOCl( g) 2NO( g) + Cl2( g) K = 1.6 1025 2NO( g) N2( g) + O2( g) K = 1 1031 For each reaction some quantities of the reactants were placed in...
-
Consider the following reaction: H2O( g) + CO(g) H2( g) + CO2(g) Amounts of H2O, CO, H2, and CO2 are put into a flask so that the composition corresponds to an equilibrium position. If the CO placed...
-
1,600 Balance Sheet The following is a list (in random order) of KIP International Products Company's December 31, 2019, balance sheet accounts: Additional Paid-In Capital on Preferred Stock $2,000...
-
Question 3 4 pts 9 x + 3 x 9 if x 0 Find a) lim f(x), b) lim, f(x), C), lim , f(x) if they exist. 3 Edit View Insert Format Tools Table : 12pt M Paragraph B IV A2 Tv
-
Mr. Geoffrey Guo had a variety of transactions during the 2019 year. Determine the total taxable capital gains included in Mr. Guo's division B income. The transactions included: 1. On January 1,...

Study smarter with the SolutionInn App


