Write the half-reactions and the balanced equation for the cell reaction for each of the following galvanic
Question:
Write the half-reactions and the balanced equation for the cell reaction for each of the following galvanic cells: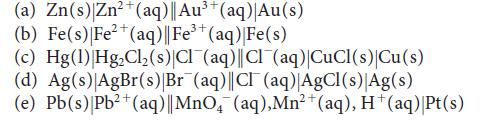
Transcribed Image Text:
2+ (a) Zn(s) Zn²+ (aq)|| Au³+ (aq)| Au(s) 3+ (b) Fe(s) | Fe²+ (aq) || Fe³+ (aq) |Fe(s) (c) Hg(1) Hg₂Cl₂ (s)|Cl(aq)||Cl(aq)|CuCl(s) Cu(s) (d) Ag(s)|AgBr(s)|Br (aq)||CI (aq)|AgCl(s)|Ag(s) (e) Pb(s) |Pb²+ (aq) || MnO₂ (aq), Mn²+ (aq), H+ (aq)|Pt(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a Zns Zn2aq Au3aq Aus Halfreactions Anode Zns Zn2aq 2e oxidation Cathode Au3aq 3e Au...View the full answer

Answered By

John Kimutai
I seek to use my competencies gained through on the job experience and skills learned in training to carry out tasks to the satisfaction of users. I have a keen interest in always delivering excellent work
4.70+
11+ Reviews
24+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Explain how the following Arduino codes affect the motion of the motor. Which direction is the motor moving? Why the map commands are required? (6%) duty1 = 40; duty2 = 60; dutyl-map...
-
Write the half reactions for the electrolysis of the elements listed in Exercise 3.
-
Write a balanced equation for each of the following reactions or reaction sequences. (a) The reaction catalyzed by PFK-2 (b) The conversion of 2 moles of oxaloacetate to glucose (c) The conversion of...
-
If A = -2 6 1 -7 1 then det (A) = an and A-1 =
-
Scrap at time of sale or at time of production, journal entries (continuation of 18-35). Assume that Job #10 of Whitefish Machine Shop generates normal scrap with a total sales value of $300 (it is...
-
For married couples living in a certain suburb, the probability that the husband will vote on a bond referendum is 0.21, the probability that his wife will vote in the referendum is 0.28, and the...
-
List common sources of risks on information technology (IT) projects? LO.1
-
Effect of Transactions on Financial Statements and Ratios the transactions listed below relate to Wainwright Inc. You are to assume that on the date on which each of the transactions occurred the...
-
ge 1: age 2: age 3: 3 Page 4: 4 Page 5: 5 Previous Page Ob) $0.75 O c) $0 Next Page Question 6 (1 point) E Listen What is the time value of a warrant with a market price of $2.25 and an exercise...
-
For R, partition the data sets into 60% training and 40% validation and implement the 10-fold cross-validation. Use the statement set. seed(1) to specify the random seed for data partitioning and...
-
The molar solubility of silver sulfite, Ag 2 SO 3 , is 1.55 * 10 5 mol L 1 . What is the K sp of silver sulfite?
-
Silver emulsion photographic film is now largely obsolete for amateur photography, but it is still used in a variety of medical and technical applications. You are working on the improvement of a...
-
A heavy chain with a mass per unit length is pulled by the constant force P along a horizontal surface consisting of a smooth section and a rough section. The chain is initially at rest on the rough...
-
What sutra is used to verify BODMAS principle in Vedic Mathematics?Explain in brief.
-
What Is Accounting? Definition, Types, History, & Examples
-
How Does Accounting Work?
-
What Are the Types of Accounting Practices?
-
The Accounting ProfessionWhat Does an Accountant Do?
-
The basal diameter of a sea anemone is an indicator of its age. The density curve shown here represents the distribution of diameters in a certain large population of anemones: the population mean...
-
Digital Fruit is financed solely by common stock and has outstanding 25 million shares with a market price of $10 a share. It now announces that it intends to issue $160 million of debt and to use...
-
Consider the couple Ox + e Red with the oxidized and redu ced species at unit activity. What must be the value of E for this half-cell if the reductant Red is to liberate hydrogen at 1 atm from a....
-
By finding appropriate half-cell reactions, calculate the equilibrium constant at 298.15 K for the following reactions: a. 4NiOOH(s) + 2 2 O(l) 4Ni(OH) 2 (s) + O 2 (g) b. 4NO 3 (aq)+ 4H + (aq)...
-
The cell potential E for the cell Pt(s)|H 2 (g, a H2 = 1) H + (aq, a H+ = 1)NaCl(aq, m = 0.300) AgCl(s) Ag(s) is +0.260 V. Determine Cl assuming that = Na+ = Cl .
-
As a Financial Analyst in the Finance Department of Zeta Auto Corporation they are seeking to expand production. The CFO asks you to help decide whether the firm should set up a new plant to...
-
Chapter 4 When an Auditor finds misstatements in entities financial statements which may be the result of fraudulent act, what should be the role of an auditor under that situation? (2 Points)
-
Suppose the following input prices are provided for each year: Required: $

Study smarter with the SolutionInn App


