Your company proposes to use a (10 mathrm{~m}^{3}) batch reactor to react methanol + acetic anhydride in
Question:
Your company proposes to use a \(10 \mathrm{~m}^{3}\) batch reactor to react methanol + acetic anhydride in a 2:1 molar ratio. The reactor has cooling coils with a maximum cooling rate of \(30 \mathrm{MJ} / \mathrm{min}\). Since the cooling is limiting, the decision was made to operate the reactor in semi-batch mode. In this mode, the methanol is first added to the reactor, then heated to the desired temperature, and the limiting reactant, acetic anhydride, is added at a constant rate to control the heat release.
The following data are available:
Reaction: \(\mathrm{CH}_{3} \mathrm{OH}+\left(\mathrm{CH}_{3} \mathrm{CO}ight)_{2} \mathrm{O}{ }^{\circledR} \mathrm{CH}_{3} \mathrm{COOCH}_{3}+\mathrm{CH}_{3} \mathrm{COOH}\) Methanol + acetic anhydride ( ) methyl acetate + acetic acid Specific gravity of final liquid mixture: 0.97 Heat of reaction: \(67.8 \mathrm{~kJ} / \mathrm{mol}\)
Additional data are also available in Examples 8-3 and 8-4.

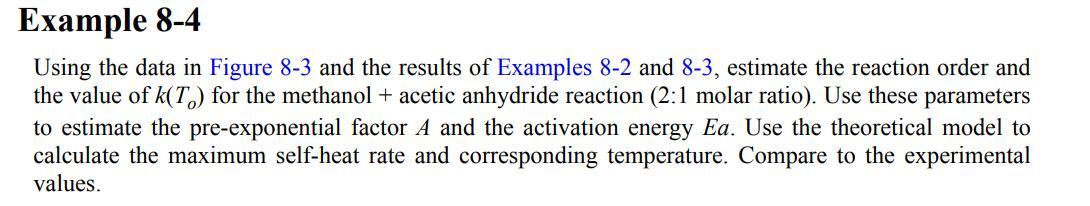
Figure 8-3
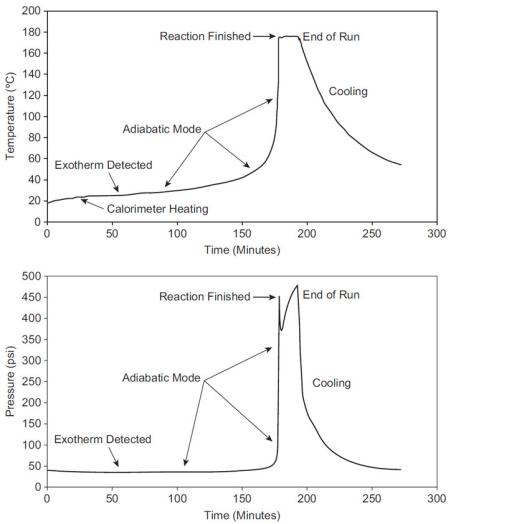
a. The company wants to produce this product as quickly as possible. To achieve this, at what temperature should it operate the reactor?
b. Assuming a maximum reactor fill factor of \(80 \%\), calculate the total amount of methanol, in \(\mathrm{kg}\), initially charged to the reactor. How much acetic anhydride, in \(\mathrm{kg}\), will be added?
c. What is the maximum addition rate of acetic anhydride, in \(\mathrm{kg} / \mathrm{min}\), to result in a reaction heat release rate equal to the cooling capacity of the cooling coils?
d. How long, in hours, will it take to do the addition?
Step by Step Answer:

Chemical Process Safety Fundamentals With Applications
ISBN: 9780134857770
4th Edition
Authors: Daniel A. Crowl, Joseph F. Louvar





